Abstract
Midkines are heparin-binding growth factors involved in a wide range of biological processes. Originally identified as retinoic acid inducible genes, midkines are widely expressed during embryogenesis with particularly high levels in the developing nervous system. During postnatal stages, midkine expression generally ceases but is often up-regulated under disease conditions, most notably those affecting the nervous system. Midkines are known as neurotrophic factors, as they promote neurite outgrowth and neuron survival in cell culture. Surprisingly, however, mouse embryos deficient for midkine (knockout mice) are phenotypically normal, which suggests functional redundancy by related growth factors. During adult stages, on the other hand, midkine knockout mice develop striking deficits in neuroprotection and regeneration after drug-induced neurotoxicity and injury. The detailed mechanisms by which midkine controls neuron formation, differentiation and maintenance remain unclear. Recent studies in zebrafish and chick have provided important insight into the role of midkine and its putative receptor, anaplastic lymphoma kinase, in cell cycle control in the central and peripheral nervous systems. A recent structural analysis of zebrafish midkine furthermore revealed essential protein domains required for biological activity that serve as promising novel targets for future drug designs. This review will summarize latest findings in the field that help to better understand the diverse roles of midkine in nervous system formation and maintenance.
Linked Articles
This article is part of a themed section on Midkine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-4
Keywords: Midkine, Pleiotrophin, neural induction, neural patterning, neurodegeneration, neuroregeneration, neuroprotection, Alk
The neural functions of midkine
Midkine (Mdk) and the related heparin-binding growth associated molecule (HB-GAM)/Pleiotrophin (Ptn) are widely expressed, heparin-binding growth factors with a molecular weight of 13 and 18 kDa respectively. They consist of functionally distinct N-terminal and C-terminal domains separated by a highly conserved hinge domain. When overexpressed, they induce pleiotropic effects described in a variety of experimental systems. The growth factor Mdk was originally identified as a retinoic acid inducible gene in embryonic carcinoma cells and initially named MK1 after its discoverers Muramatsu and Kadomatsu (Kadomatsu et al., 1988). Later it was renamed to midkine (Mdk), as it was characterized as a cytokine being expressed during mid-gestation periods of mammalian embryos (Tsutsui et al., 1991). Mdk is widely expressed in mouse embryos, with elevated and developmentally regulated patterns in brain and spinal cord, but strongly down-regulated shortly after birth, persisting only in the kidney. It was first demonstrated for Ptn/HB-GAM and then later for Mdk that both growth factors promoted neurite outgrowth (Rauvala, 1989; Maruta et al., 1993; Muramatsu et al., 1993). In addition, Mdk was shown to have neurotrophic activity in cultured spinal cord and dorsal root ganglion neurons derived from fetal mice (Michikawa et al., 1993). Using grid assays, where recombinant Mdk protein was applied in array stripes onto culture dishes, rat brain derived neurons preferentially grew their neurites along the Mdk-coated trails (Kaneda et al., 1996). Importantly, this adhesion between neurite surface and Mdk-coated matrix was efficiently blocked when heparin was added to the culture medium. This strongly suggested that extracellular Mdk serves as a growth promoting cue driving matrix adhesion and outgrowth of neurites in a heparin-dependent manner (Kaneda et al., 1996). Furthermore, delivery of beads coated with recombinant Mdk to cultures of muscle cells induced clustering of acetylcholine receptors (Zhou et al., 1997). Such neurotransmitter receptor clustering in target cells is characteristic of the establishment of neuromuscular junctions and indicates formation of active synapses. Receptor clustering triggered by Mdk thus suggested a possible contribution of Mdk to synapse formation, which was further supported by the detection of endogenous Mdk protein at presynaptic terminals (Zhou et al., 1997). In addition, several studies reported that Mdk and Ptn acted as survival factors for neurons (Kikuchi et al., 1993; Satoh et al., 1993; Hida et al., 2003) and that this activity was mediated through inhibition of apoptosis by modulating the MAPK pathway (Owada et al., 1999). A more recent study in chicks reported that Mdk controlled proliferation of sympathetic neurons in the peripheral nervous system (PNS; Reiff et al., 2011). These authors showed that Mdk was expressed in neuronal, as well as non-neuronal cells of sympathetic ganglia during proliferative stages, and controlled the maturation of sympathetic neuron progenitors derived from the neural crest. Inhibition of Mdk or its putative receptor anaplastic lymphoma kinase (Alk) resulted in reduced proliferation and induced apoptosis, hence further supporting the role of Mdk as survival factor for neurons and a crucial factor for neurogenesis in vivo (Reiff et al., 2011). So far, the vast majority of studies characterized embryonic neural activities of Mdk in cell culture systems, with some exceptions (see Reiff et al., 2011). Therefore, the generation of mutant animal models was expected to provide important new insights by validating cell culture data in vivo and determining whether Mdk-deficient animals show any neurological defects.
Studying neural function in the Mdk knockout mouse
To assess the function of Mdks in vivo, knockout mice were generated. Despite the widespread embryonic expression of Mdk and Ptn, and potent activities observed in cell culture assays, Mdk-, as well as Ptn-deficient mice develop with no gross phenotypical abnormalities (Nakamura et al., 1998; Amet et al., 2001). Functional redundancy was therefore postulated to account for the absence of obvious phenotypes (Muramatsu, 2002). Consistent with this hypothesis, a 230-fold up-regulation of Ptn transcription in Mdk knockout mice was observed, notably in an organ-specific manner (Herradon et al., 2005). Moreover, the majority of double knockout mice deficient for both Mdk and Ptn die in the early embryonic stages (Muramatsu et al., 2006). Only few doubly deficient mice are viable and most of the viable females become infertile. Double knockout mice that are not viable unfortunately die before an assessment of neural abnormalities is possible. Thus, so far no early neural phenotypes have been described in these Mdk-/-/Ptn-/- mice.
More thorough analysis of Mdk-/- and Ptn-/- single knock-out mice, however, revealed several defects in the nervous system at the molecular and behavioural level during later stages of life. Although embryonic development proceeded phenotypically normal in Mdk-/- mice, expression changes were recorded at postnatal stages (Nakamura et al., 1998). Calretinin expression in the dentate gyrus granule cell layer of the hippocampus was up-regulated in postnatal brains, and Mdk-/- mice subsequently also exhibited deficits in memory and increased anxiety, assessed in maze tests (Nakamura et al., 1998). This was consistent with findings in Ptn-/- mice, where an increase in long-term potentiation due to lowered induction thresholds was reported in the hippocampus (Amet et al., 2001). Furthermore, levels of dopamine and its receptors were reduced in the striatum of Mdk-/- mice (Ohgake et al., 2009). Consistent with this, a more recent study identified typical preclinical features of Parkinson's disease in Mdk deficient mice (Prediger et al., 2011). These authors observed partial depletion of mesencephalic dopaminergic neurons, reduced dopamine levels in olfactory bulb and striatum together with impaired olfactory discrimination and short-term memory in Mdk-/- mice. This strongly suggested significant deficits in dopaminergic neurotransmission in the absence of Mdk and suggested Mdk deficient mice as a possible model to analyse preclinical stages of Parkinson's disease (Prediger et al., 2011). Another important study furthermore showed that morphologically normal, Mdk knockout mice show significant alterations of drug-induced neurotoxicity and addictive behaviour (Gramage et al., 2011).
Together, all these findings provide strong evidence suggesting that Mdk and Ptn play important roles in neurotransmission and the maintenance and plasticity of neuronal connections in the postnatal and adult nervous system. Yet, the embryonic neural functions of Mdk remain a mystery. The absence of obvious early neural phenotypes in Mdk-/- and Ptn-/- knockout embryos remains puzzling given the widespread expression of these growth factors during early embryonic stages, and the potent activities of these growth factors on cultured neurons in neurite outgrowth assays. Therefore, alternative animal models were analysed for Mdk loss of function phenotypes, including the zebrafish as a powerful vertebrate model organism.
Characterization of the neural function of Mdk in the zebrafish model
The zebrafish has become a popular vertebrate model for biomedical research and drug discovery (Peterson and Macrae, 2012). It offers several experimental features that make it unique for efficient functional gene analysis during vertebrate organogenesis. These features include the possibility of combining live imaging in intact specimens with powerful genetic approaches, such as large scale mutagenesis. Furthermore, zebrafish produce abundant embryos that are almost completely transparent and develop rapidly outside the mother. Zebrafish Mdk was first isolated in a cross-species expression cloning screen for neural inducing factors using the Xenopus laevis animal cap assay (Winkler and Moon, 2001). In this screen, large cDNA pools from a zebrafish adult brain library containing 25 000 clones were transcribed into mRNA and injected into two-cell stage Xenopus embryos. The injected embryos were incubated until the blastula stage, then animal cap tissue was explanted and cultivated in vitro. Neural induction was assessed by determining neural marker gene expression in the absence of mesoderm formation. Once a positive cDNA pool was identified, it was subdivided into progressively smaller pools using sib selection. This procedure was repeated until less complex pools and eventually individual clones were identified that showed neural inducing activity. After six rounds of sib selection, a single clone was identified that robustly induced expression of pan neural markers (Nrp, Ncam) and the cement gland marker Xag1 in the absence of mesoderm, suggesting that neural induction was direct and that this factor converted naïve ectoderm into neural-fated tissue (Winkler and Moon, 2001). Sequencing of this clone revealed a remarkable sequence homology to human Mdk (67% amino acid identity), and therefore the identified gene was named zebrafish mdk2. Shortly after this discovery, cDNA screening identified a second Mdk orthologue in the genome of zebrafish. Sequence alignment and synteny analysis revealed that this second copy was different from Ptn but represented a duplicated Mdk gene copy. This analysis suggested that both zebrafish Mdk genes originated by a fish specific genome duplication (FSGD) that occurred in evolution shortly after the separation of the teleost from the tetrapod lineage approximately 300 million years ago (Figure 1A; Winkler et al., 2003). Expression and functional analysis revealed that both zebrafish Mdk genes, now named mdka and mdkb according to the zebrafish gene nomenclature, underwent significant functional divergence during evolution (Figure 1B,C; Table 1). At this point, the zebrafish Mdk genes represented one of the first examples demonstrating the principle of subfunction partitioning after FSGD, a process that strongly contributed to the rapid evolution and radiation of teleost fishes (Winkler et al., 2003; Meyer and Van de Peer, 2005).
Figure 1.
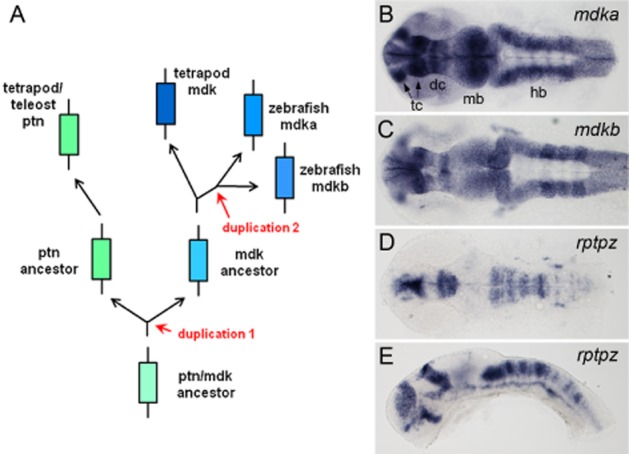
Evolution of the Mdk/Ptn family and divergence of zebrafish mdka and mdkb after gene duplication. (A) mdk and ptn arose from a gene duplication that occurred before the separation from tetrapods and teleost fish (duplication 1). The two zebrafish mdk co-orthologs arose from a teleost specific genome duplication (duplication 2). There is only one Ptn gene known in zebrafish, but it is possible that duplicated Ptn genes are present in other teleost fish; arrow lengths are not drawn to scale. (B, C) Expression of mdka and mdkb in the developing zebrafish brain at 24 hours post fertilization (hpf), as analysed by RNA whole-mount in situ hybridization. Note expression differences in telencephalon (tc), diencephalon (dc), midbrain (mb) and hindbrain (hb). (D, E) Expression of the putative Mdk receptor rptpz in the developing zebrafish brain at 24 hpf. rptpz is expressed in domains directly adjacent to or overlapping with those expressing mdka or mdkb. B, C, D are dorsal views with anterior to the left, E is a lateral view.
Table.
Expression, knock-down and overexpression phenotypes of zebrafish and mouse Mdk orthologues during embryogenesis
| Zebrafish mdka | Zebrafish mdkb | Mouse Mdk | |
|---|---|---|---|
| RNA expression during early embryogenesis (zebrafish: <24 hpf; mouse: <E9) | Paraxial mesoderm, brain, medial spinal cord; retinal stem and progenitor cells | Neural plate borders, brain, dorsal spinal cord; differentiating retinal neurons | Ubiquitous |
| Embryonic knock-down phenotype | Expanded notochord, reduced floor plate; reduced proliferation in retina, microphthalmia | Reduced numbers of neural crest cells and sensory neurons | No phenotype |
| Embryonic overexpression phenotype | Expanded floor plate, reduced notochord, absent somite boundaries; retinal overgrowth, cyclopia | Expanded neural crest and increased numbers of sensory neurons; microphthalmia | Not tested |
| References | Winkler et al., 2003; Schafer et al., 2005; Calinescu et al., 2009; Luo et al., 2012; Lim et al., 2013 | Winkler et al., 2003; Liedtke and Winkler, 2008; Calinescu et al., 2009; Lim et al., 2013 | Kadomatsu et al., 1988, 1990; Nakamoto et al., 1992 |
Using gene knockdown and overexpression approaches, it was shown that mdka and mdkb play strictly different roles in patterning the early nervous system in zebrafish. Both zebrafish Mdk genes are expressed in mostly non-overlapping patterns in the developing nervous system (Table 1, Figure 1B,C; Winkler et al., 2003). Mdka controls formation of the medial floor plate (MFP), an important organizing centre for the early nervous system, which secretes sonic hedgehog (Shh) and establishes polarity in the developing brain and spinal cord (Schafer et al., 2005). A deficiency in mdka resulted in the absence of the MFP, while the number of notochord cells was increased. This suggested that Mdka is involved in fate decisions between both cell types during gastrulation. Based on these findings, a novel mechanism for floor plate formation in vertebrates was proposed, which implicated that trunk-derived Mdka forces prespecified, organizer-derived axial mesoderm cells into the ventral midline of the developing spinal cord (Schafer et al., 2005). mdkb, on the other hand, is expressed in a pattern that is mostly complementary to that of mdka. At neural plate stages, expression levels are high at the neural plate boundaries separating neural plate ectoderm from non-neural epidermal ectoderm (Winkler et al., 2003). At later stages, mdkb is strongly expressed in the dorsal spinal cord. Gene knockdown of mdkb resulted in the absence of neural crest cells and sensory neurons (Liedtke and Winkler, 2008). This shows that mdka and mdkb play crucial roles during formation and patterning of the embryonic nervous system in zebrafish.
A Ptn/HB-GAM orthologue has also been identified in the zebrafish genome (Chang et al., 2004). It is expressed in a pattern that is strikingly different from that of mdka and mdkb, but its detailed embryonic function remains unclear (D. Liedtke and C. Winkler, unpubl. data). Like its mammalian counterpart, however, zebrafish Ptn also induced neurite outgrowth in PC12 cells without NGF stimulation as well as enhancing neuritogenesis in zebrafish embryos (Chang et al., 2004).
Interestingly and in contrast to higher vertebrates, zebrafish mdk and ptn are expressed at high levels in the adult brain in spatially restricted domains (Winkler et al., 2003; Chang et al., 2004). It is tempting to speculate that maintenance of Mdk expression in adult neural tissue can be linked to the capacity of teleosts to efficiently regenerate experimentally induced lesions induced in the CNS (Kizil et al., 2012; Reimer et al., 2013).
Mdks in neural protection, regeneration and cell cycle control
Mdk and Ptn have been described as neurotrophic factors promoting the survival and function of neurons (Unoki et al., 1994; Masuda et al., 1995; Prediger et al., 2011). They are up-regulated in a variety of neurodegenerative disorders, such as Parkinson's disease (Marchionini et al., 2007), and have been assigned protective activities as response to drug-induced neurodegeneration (Gramage and Herradon, 2011). Experimentally induced overexpression of Mdk and Ptn also showed neuroprotective effects in various mouse models for Parkinson's disease and Alzheimer's disease (see Muramatsu, 2011). Conversely, a Mdk deficiency results in a delay of neural degeneration and regeneration of the PNS (Sakakima et al., 2009). Injury imposed on the peripheral sciatic nerve in wild-type and Mdk-/- mice revealed a significant delay in nerve regeneration and recovery of the associated soleus muscle when Mdk was absent (Sakakima et al., 2009). Most interestingly, a recent report has shown that exogenously applied Mdk even promoted neural regeneration in the injured mouse spinal cord in vivo (Muramoto et al., 2013). This is especially remarkable, as regenerative processes in the CNS are usually efficiently inhibited by activated glial cells. Noteworthy, endogenous Mdk expression was induced after spinal cord injury (Sakakima et al., 2004), suggesting the presence of an intrinsic mechanism in mammals to overcome glial-induced inhibitory processes and allow neuronal axon regeneration.
The positive effect of Mdk or Ptn on regeneration is also documented by several other studies that accumulated over the last decade, most notably for the nervous system. Ptn has been shown to be involved in regenerative processes of the brain (Iseki et al., 2002), while Mdk is associated with regeneration in the PNS (Sakakima et al., 2009) and the retina (Calinescu et al., 2009). The architecture of the retina and organization of its functional circuits are remarkably conserved during vertebrate evolution allowing the use of animal models to study disease-related processes known in humans (Fadool and Dowling, 2008). Intravitreal injection of Mdk protein into rats exposed to constant light flash stimulation for 1 week efficiently protected photoreceptor cells from damage, as recorded at the morphological (Unoki et al., 1994), as well as physiological level (Masuda et al., 1995), notably however without regeneration of new photoreceptors. In contrast to mammals, zebrafish are able to regenerate lost photoreceptor neurons (Vihtelic and Hyde, 2000), but also other tissues, including neurons and axons in the CNS (Reimer et al., 2013). This opens the possibility of analysing Mdk's function during regeneration in vivo using the zebrafish model. Regeneration of damaged tissues involves re-entry of quiescent progenitor cells into the cell cycle. Interestingly, both zebrafish Mdk genes were strongly up-regulated in retinal stem cells during regeneration of photoreceptor cells as they re-entered the cell cycle (Calinescu et al., 2009). A more recent study has now shown that zebrafish Mdka controls the cell cycle during neurogenesis of photoreceptors (Luo et al., 2012). Both zebrafish Mdks, mdka and mdkb, are dynamically expressed in the zebrafish retina, albeit with different patterns (Calinescu et al., 2009). While mdka is expressed in retinal stem and progenitor cells (i.e. cells of the ciliary marginal zone and Muller glial cells), mdkb is expressed in newly post-mitotic cells shortly after they have exited the cell cycle to differentiate into various retinal neurons such as ganglion and amacrine cells (Calinescu et al., 2009). Expression of mdkb is excluded from progenitor cells. Gene knockdown studies in zebrafish embryos showed that mdka is required for cell cycle exit during formation of retinal photoreceptors. A deficiency in mdka results in microphthalmia caused by delayed neuronal differentiation as fewer progenitor cells exit the cell cycle (Luo et al., 2012). In contrast, ectopic overexpression of mdka accelerated the cell cycle and resulted in transient retinal overgrowth. Using various cell cycle markers, Luo et al. (2012) concluded that mdka levels regulate the progression from S-to M-phase of the cell cycle. Although corresponding data for mdkb are still lacking, this suggests that the two zebrafish Mdk co-orthologues have evolved distinct expression patterns reflecting possibly divergent activities in controlling cell cycle kinetics of neuronal progenitors. The tightly controlled regulation of two zebrafish Mdk genes in stem cells versus differentiated cells has important implications for the situation in other vertebrates. In mammals, only one Mdk gene exists. It thus remains open whether Mdk in mammals has similar functions in regulating cell cycle exit and differentiation of stem or progenitor cells. It will be interesting to find out whether the strictly distinct roles of the two zebrafish orthologues during cell cycle exit are reproduced by the single Mdk protein in mammals through interaction with different co-factors or receptors. As Mdk genes are highly expressed in the adult brain of zebrafish under normal conditions (Winkler et al., 2003), as well as under disease conditions in mammals, it is furthermore tempting to speculate that Mdk might be involved in the differentiation of neural progenitor cells in the embryonic and adult CNS of zebrafish and mammals, similar to the situation found in embryonic photoreceptors.
The structural basis of Mdk function
Mdk and Ptn are proteins rich in basic aminoacids, with evolutionarily highly conserved cysteine residues. In zebrafish, the duplicated mdka and mdkb genes encode proteins that share 65% sequence identity (62.3% and 58.2% identity to human Mdk, respectively; mature protein without signal peptide), but show significant functional divergence during nervous system formation (Table 1; Schafer et al., 2005; Liedtke and Winkler, 2008) and in the developing retina (Luo et al., 2012). This divergence offers a unique opportunity to analyse in vivo the effects of single amino acid substitutions in the two related sequences on protein structure and ultimately, biological function. The NMR structures of individual N-terminal and C-terminal domains of human Mdk have been previously reported (Iwasaki et al., 1997), and the C-terminal domain is a popular therapeutic target (see Muramatsu, 2011). Recently, the NMR structure of the full-length zebrafish Mdkb protein was obtained (Lim et al., 2013). Site-directed mutagenesis revealed residues that are critical for structural features, heparin-binding and biological activity. The highly conserved hinge region separating the N-and C-terminal domains has previously been proposed to confer flexibility to the full-length protein. The study by Lim et al. (2013) surprisingly showed that the hinge domain itself binds to heparin and is essential for coordinating simultaneous binding of N-and C-terminal domains to heparin. This opens the possibility that the hinge region, so far basically ignored as a potential drug target, could be used for future drug design and therapeutic interventions. The present zebrafish study also demonstrated that certain aspects of Mdkb's biological activity in embryos were not strictly dependent on heparin binding. This observation could also lead to possibly novel drug design strategies that aim at biologically relevant residues directly, rather than at heparin-binding domains. Other than previously observed in cell culture studies (Maeda et al., 1999), expression of the C-terminal domain alone does not exert any biological effect in vivo in zebrafish embryos. Instead, hinge and N-terminal domains together with the C-terminal domain are required to confer efficient heparin-binding and biological activity. The present structure-function analysis for Mdk by Lim et al. (2013) thus demonstrates the value of simple in vivo models to analyse the full range of biological effects of Mdk variants. The zebrafish model furthermore offers unique opportunities for in vivo drug screening of potential Mdk inhibitors. At present, studies are ongoing to determine the full-length structure of zebrafish Mdka. A comparison of Mdka and Mdkb structures and their corresponding biological activities is likely to identify individual motifs responsible for distinct structural configurations. These in turn are expected to be responsible for the divergent biological activates observed, for example through interaction with different receptor complexes and activation of specific signal transduction pathways.
The receptors mediating the neural functions of Mdk
Previous studies performed mainly in cell culture suggested that Mdks bind to several cell surface receptors. Mdk and Ptn both induce phosphorylation of the receptor tyrosine kinase Alk and its downstream kinase PI3K suggesting at least an indirect interaction between Mdk and Alk (Stoica et al., 2001; 2002,). Other putative transmembrane Mdk receptors include the receptor protein tyrosine phosphatase ζ (RPTPζ; Maeda et al., 1999), integrins (Muramatsu et al., 2004) and low-density lipoprotein-receptor related protein (Muramatsu et al., 2000). Whether Mdk binds and activates Alk directly or indirectly through RPTPζ, and whether Mdk and Ptn exhibit similar binding kinetics remains under discussion (Perez-Pinera et al., 2007).
In zebrafish, experimentally induced overexpression of Alk leads to activation of the MEK/ERK pathway, increased cell proliferation in the CNS and aberrant neurogenesis leading to mispositioning of differentiated neurons (Yao et al., 2013). Interestingly, a knockdown of Alk in early zebrafish embryos did not affect formation of neural progenitor and stem cells, but prevented their differentiation into mature CNS neurons suggesting that cell cycle exit of the neural progenitors is disturbed. This is consistent with findings in the PNS in chicks (Reiff et al., 2011) and suggests that Alk is a general regulator of neurogenesis in the CNS and PNS of vertebrates. This proposed role for Alk in cell cycle exit is also remarkably similar to that proposed for Mdk function in the zebrafish retina (Luo et al., 2012), providing possible support for an expected Mdk-Alk interaction. Importantly, however, the overall knockdown phenotypes for Alk and Mdk in zebrafish are strikingly different. Knockdown of mdka and mdkb leads to patterning defects in the zebrafish neural plate as early as during gastrulation (Schafer et al., 2005; Liedtke and Winkler, 2008), while inhibition of Alk only affects neurogenesis at much later stages when patterning is already completed (Yao et al., 2013).
While Alk has been proposed as a potential receptor for Mdk in mammals, a direct interaction of Mdk with Alk in zebrafish or Drosophila remains unclear. Notably, the physiological ligand for Alk in Drosophila, Jelly Belly (Jeb; Englund et al., 2003; Lee et al., 2003) shares no sequence or structural homology with the Mdk homologue in Drosophila (Miple) or vertebrate Mdk, and is not capable of activating mouse Alk (Yang et al., 2007). Also in zebrafish, a direct binding of Mdk to Alk appears highly unlikely. Similar to mdk and ptn, alk and rptpζ are also expressed strongly in the developing CNS of zebrafish, often with overlapping patterns (Figure 1D,E; Winkler et al., 2003; Yao Sheng, data not shown; Yao et al., 2013). Notably, however, there is significant sequence divergence found in the extracellular domain of Alk in vertebrates. While Mdk is highly conserved at the amino acid level between zebrafish and human (62.3% amino acid identity for Mdka), as is the kinase domain of Alk (89.6%), the identity in the extracellular ligand-binding domain of Alk is strikingly low (25.3%). This profound sequence divergence in the Alk ligand-binding domain argues against a direct Mdk-Alk interaction, which is consistent with the different effects observed after knockdown of both components in zebrafish embryos in vivo. This suggests that Mdk might bind to other receptors in the developing nervous system. While this could argue against a direct interaction of Mdk and Alk in vertebrates in general, it does not exclude the possibility that Mdk regulates Alk indirectly, for example through binding to RPTPζ. This idea has been proposed earlier based on cell culture experiments (Perez-Pinera et al., 2007). Interestingly, zebrafish rptpζ shows a spatially and temporally highly dynamic expression profile, which is in remarkable contrast to the more widespread expression of alk (Figure 1D,E; Yao et al., 2013). It is therefore tempting to speculate that RPTPζ mediates the regionally restricted effects observed after constitutive Mdk or Alk overexpression.
Clearly, more studies are needed to elucidate the developmental roles of Mdk-receptor complexes and gain insight into the composition of probably multicomponent receptor complexes. It appears likely that the Mdk receptor is an assembly of several proteins including Alk, Lrp6, RPTPζ, integrins and others, as proposed earlier (Sakaguchi et al., 2003). Interestingly, a recent study has shown that Drososphila Alk controls the activity of Gli transcriptional activators and repressors (Popichenko et al., 2013). Gli factors on the other hand are known as direct downstream targets of Shh signalling, which is crucial for establishing the early nervous system in vertebrates (Jacob and Briscoe, 2003). This opens the attractive possibility that Mdk-Alk signalling cross-talks with Shh signalling components during embryogenesis to establish distinct neural fates in the embryonic brain and spinal cord.
Conclusion and outlook
The Mdk growth factors are widely expressed during embryogenesis, but in adult tissues show elevated levels mostly under disease, injury and repair conditions. They have been assigned neurotrophic and neuroprotective roles, in particular in context of drug-induced neurotoxicity, nerve injury and neurodegeneration at later stages of life. Thus, while multiple roles in the postnatal and adult nervous system have been firmly established, their role during embryonic neurogenesis remains less clear. Functional redundancy with other growth factors has been made responsible for the fact that Mdk-deficient embryos develop normally with no gross morphological abnormalities. Such redundancy could also be relevant at adult stages and thus could mask additional aspects of Mdk function that have remained unknown so far. Until now, no attempts have been reported to generate conditional mouse mutants where Mdk and Ptn are simultaneously inactivated in individual tissues and/or at distinct stages. This could solve the problem of early lethality of double knockout mice and reveal so far unidentified cell type-specific Mdk functions.
A detailed analysis of the endogenous roles of Mdk during neurogenesis and neural differentiation using conditional knockout mice or alternative animal models such as zebrafish is expected to provide a better understanding of the many roles of Mdk during nervous system formation and function. Assigning individual functional aspects to particular structural features of this relatively small growth factor will help to establish Mdk and Ptn as better drug targets, for example to enhance their neuroprotective capacities. Of particular importance for drug design will be the description of structural changes that are induced after Mdk has bound to its different cellular receptors. A better understanding of the mechanisms involved in Mdk-receptor interaction at a structural and functional level will provide the basis for design of tailored drugs that interfere with Mdk activity in a variety of pathological conditions ranging from neurodegenerative diseases to inflammation and cancer.
Acknowledgments
The authors want to thank Daniel Liedtke, Matthias Schafer, Lim Jack Wee and Yang Daiwen for fruitful discussions. This work is supported by a Singapore Ministry of Education-Academic Research Fund grant (R-154-000-478-112) to C. W.
Glossary
- Alk
anaplastic lymphoma kinase
- FSGD
fish specific genome duplication
- HB-GAM
heparin-binding, growth associated molecule
- Mdk
midkine
- MFP
medial floor plate
- PNS
peripheral nervous system
- Ptn
pleiotrophin
- RPTP
receptor phospho-tyrosine phosphatase
- Shh
Sonic hedgehog
Conflict of interest
The authors declare to have no conflict of interest.
References
- Amet LE, Lauri SE, Hienola A, Croll SD, Lu Y, Levorse JM, et al. Enhanced hippocampal long-term potentiation in mice lacking heparin-binding growth-associated molecule. Mol Cell Neurosci. 2001;17:1014–1024. doi: 10.1006/mcne.2001.0998. [DOI] [PubMed] [Google Scholar]
- Calinescu AA, Vihtelic TS, Hyde DR, Hitchcock PF. Cellular expression of midkine-a and midkine-b during retinal development and photoreceptor regeneration in zebrafish. J Comp Neurol. 2009;514:1–10. doi: 10.1002/cne.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MH, Huang CJ, Hwang SP, Lu IC, Lin CM, Kuo TF, et al. Zebrafish heparin-binding neurotrophic factor enhances neurite outgrowth during its development. Biochem Biophys Res Commun. 2004;321:502–509. doi: 10.1016/j.bbrc.2004.06.172. [DOI] [PubMed] [Google Scholar]
- Englund C, Loren CE, Grabbe C, Varshney GK, Deleuil F, Hallberg B, et al. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature. 2003;425:512–516. doi: 10.1038/nature01950. [DOI] [PubMed] [Google Scholar]
- Fadool JM, Dowling JE. Zebrafish: a model system for the study of eye genetics. Prog Retin Eye Res. 2008;27:89–110. doi: 10.1016/j.preteyeres.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramage E, Herradon G. Connecting Parkinson's disease and drug addiction: common players reveal unexpected disease connections and novel therapeutic approaches. Curr Pharm Des. 2011;17:449–461. doi: 10.2174/138161211795164103. [DOI] [PubMed] [Google Scholar]
- Gramage E, Martin YB, Ramanah P, Perez-Garcia C, Herradon G. Midkine regulates amphetamine-induced astrocytosis in striatum but has no effects on amphetamine-induced striatal dopaminergic denervation and addictive effects: functional differences between pleiotrophin and midkine. Neuroscience. 2011;190:307–317. doi: 10.1016/j.neuroscience.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Herradon G, Ezquerra L, Nguyen T, Silos-Santiago I, Deuel TF. Midkine regulates pleiotrophin organ-specific gene expression: evidence for transcriptional regulation and functional redundancy within the pleiotrophin/midkine developmental gene family. Biochem Biophys Res Commun. 2005;333:714–721. doi: 10.1016/j.bbrc.2005.05.160. [DOI] [PubMed] [Google Scholar]
- Hida H, Jung CG, Wu CZ, Kim HJ, Kodama Y, Masuda T, et al. Pleiotrophin exhibits a trophic effect on survival of dopaminergic neurons in vitro. Eur J Neurosci. 2003;17:2127–2134. doi: 10.1046/j.1460-9568.2003.02661.x. [DOI] [PubMed] [Google Scholar]
- Iseki K, Hagino S, Mori T, Zhang Y, Yokoya S, Takaki H, et al. Increased syndecan expression by pleiotrophin and FGF receptor-expressing astrocytes in injured brain tissue. Glia. 2002;39:1–9. doi: 10.1002/glia.10078. [DOI] [PubMed] [Google Scholar]
- Iwasaki W, Nagata K, Hatanaka H, Inui T, Kimura T, Muramatsu T, et al. Solution structure of midkine, a new heparin-binding growth factor. EMBO J. 1997;16:6936–6946. doi: 10.1093/emboj/16.23.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Briscoe J. Gli proteins and the control of spinal-cord patterning. EMBO Rep. 2003;4:761–765. doi: 10.1038/sj.embor.embor896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadomatsu K, Tomomura M, Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun. 1988;151:1312–1318. doi: 10.1016/s0006-291x(88)80505-9. [DOI] [PubMed] [Google Scholar]
- Kadomatsu K, Huang RP, Suganuma T, Murata F, Muramatsu T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J Cell Biol. 1990;110:607–616. doi: 10.1083/jcb.110.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda N, Talukder AH, Nishiyama H, Koizumi S, Muramatsu T. Midkine, a heparin-binding growth/differentiation factor, exhibits nerve cell adhesion and guidance activity for neurite outgrowth in vitro. J Biochem. 1996;119:1150–1156. doi: 10.1093/oxfordjournals.jbchem.a021361. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Muramatsu H, Muramatsu T, Kim SU. Midkine, a novel neurotrophic factor, promotes survival of mesencephalic neurons in culture. Neurosci Lett. 1993;160:9–12. doi: 10.1016/0304-3940(93)90904-y. [DOI] [PubMed] [Google Scholar]
- Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2012;72:429–461. doi: 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- Lee HH, Norris A, Weiss JB, Frasch M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature. 2003;425:507–512. doi: 10.1038/nature01916. [DOI] [PubMed] [Google Scholar]
- Liedtke D, Winkler C. Midkine-b regulates cell specification at the neural plate border in zebrafish. Dev Dyn. 2008;237:62–74. doi: 10.1002/dvdy.21384. [DOI] [PubMed] [Google Scholar]
- Lim J, Yao S, Graf M, Winkler C, Yang D. Structure-function analysis of full-length midkine reveals novel residues important for heparin binding and zebrafish embryogenesis. Biochem J. 2013;451:407–415. doi: 10.1042/BJ20121622. [DOI] [PubMed] [Google Scholar]
- Luo J, Uribe RA, Hayton S, Calinescu AA, Gross JM, Hitchcock PF. Midkine-A functions upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate retina. Neural Dev. 2012;7:33. doi: 10.1186/1749-8104-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- Marchionini DM, Lehrmann E, Chu Y, He B, Sortwell CE, Becker KG, et al. Role of heparin binding growth factors in nigrostriatal dopamine system development and Parkinson's disease. Brain Res. 2007;1147:77–88. doi: 10.1016/j.brainres.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Maruta H, Bartlett PF, Nurcombe V, Nur EKMS, Chomienne C, Muramatsu T, et al. Midkine (MK), a retinoic acid (RA)-inducible gene product, produced in E. coli acts on neuronal and HL60 leukemia cells. Growth Factors. 1993;8:119–134. doi: 10.3109/08977199309046932. [DOI] [PubMed] [Google Scholar]
- Masuda K, Watanabe I, Unoki K, Ohba N, Muramatsu T. Functional rescue of photoreceptors from the damaging effects of constant light by survival-promoting factors in the rat. Invest Ophthalmol Vis Sci. 1995;36:2142–2146. [PubMed] [Google Scholar]
- Meyer A, Van de Peer Y. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD) Bioessays. 2005;27:937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- Michikawa M, Kikuchi S, Muramatsu H, Muramatsu T, Kim SU. Retinoic acid responsive gene product, midkine, has neurotrophic functions for mouse spinal cord and dorsal root ganglion neurons in culture. J Neurosci Res. 1993;35:530–539. doi: 10.1002/jnr.490350509. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Shirahama H, Yonezawa S, Maruta H, Muramatsu T. Midkine, a retinoic acid-inducible growth/differentiation factor: immunochemical evidence for the function and distribution. Dev Biol. 1993;159:392–402. doi: 10.1006/dbio.1993.1250. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Zou K, Sakaguchi N, Ikematsu S, Sakuma S, Muramatsu T. LDL receptor-related protein as a component of the midkine receptor. Biochem Biophys Res Commun. 2000;270:936–941. doi: 10.1006/bbrc.2000.2549. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Zou P, Suzuki H, Oda Y, Chen GY, Sakaguchi N, et al. alpha4beta1-and alpha6beta1-integrins are functional receptors for midkine, a heparin-binding growth factor. J Cell Sci. 2004;117(Pt 22):5405–5415. doi: 10.1242/jcs.01423. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Zou P, Kurosawa N, Ichihara-Tanaka K, Maruyama K, Inoh K, et al. Female infertility in mice deficient in midkine and pleiotrophin, which form a distinct family of growth factors. Genes Cells. 2006;11:1405–1417. doi: 10.1111/j.1365-2443.2006.01028.x. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Midkine: a promising molecule for drug development to treat diseases of the central nervous system. Curr Pharm Des. 2011;17:410–423. doi: 10.2174/138161211795164167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto A, Imagama S, Natori T, Wakao N, Ando K, Tauchi R, et al. Midkine overcomes neurite outgrowth inhibition of chondroitin sulfate proteoglycan without glial activation and promotes functional recovery after spinal cord injury. Neurosci Lett. 2013;550:150–155. doi: 10.1016/j.neulet.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Matsubara S, Miyauchi T, Obama H, Ozawa M, Muramatsu T. A new family of heparin binding growth/differentiation factors: differential expression of the midkine (MK) and HB-GAM genes during mouse development. J Biochem. 1992;112:346–349. doi: 10.1093/oxfordjournals.jbchem.a123903. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Kadomatsu K, Yuasa S, Muramatsu H, Mamiya T, Nabeshima T, et al. Disruption of the midkine gene (Mdk) resulted in altered expression of a calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells. 1998;3:811–822. doi: 10.1046/j.1365-2443.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Ohgake S, Shimizu E, Hashimoto K, Okamura N, Koike K, Koizumi H, et al. Dopaminergic hypofunctions and prepulse inhibition deficits in mice lacking midkine. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:541–546. doi: 10.1016/j.pnpbp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Owada K, Sanjo N, Kobayashi T, Mizusawa H, Muramatsu H, Muramatsu T, et al. Midkine inhibits caspase-dependent apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase in cultured neurons. J Neurochem. 1999;73:2084–2092. [PubMed] [Google Scholar]
- Perez-Pinera P, Zhang W, Chang Y, Vega JA, Deuel TF. Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase beta/zeta signaling pathway: an alternative mechanism of receptor tyrosine kinase activation. J Biol Chem. 2007;282:28683–28690. doi: 10.1074/jbc.M704505200. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Macrae CA. Systematic approaches to toxicology in the zebrafish. Annu Rev Pharmacol Toxicol. 2012;52:433–453. doi: 10.1146/annurev-pharmtox-010611-134751. [DOI] [PubMed] [Google Scholar]
- Popichenko D, Hugosson F, Sjogren C, Dogru M, Yamazaki Y, Wolfstetter G, et al. Jeb/Alk signalling regulates the Lame duck GLI family transcription factor in the Drosophila visceral mesoderm. Development. 2013;140:3156–3166. doi: 10.1242/dev.094466. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Rojas-Mayorquin AE, Aguiar AS, Jr, Chevarin C, Mongeau R, Hamon M, et al. Mice with genetic deletion of the heparin-binding growth factor midkine exhibit early preclinical features of Parkinson's disease. J Neural Transm. 2011;118:1215–1225. doi: 10.1007/s00702-010-0568-3. [DOI] [PubMed] [Google Scholar]
- Rauvala H. An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J. 1989;8:2933–2941. doi: 10.1002/j.1460-2075.1989.tb08443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff T, Huber L, Kramer M, Delattre O, Janoueix-Lerosey I, Rohrer H. Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. Development. 2011;138:4699–4708. doi: 10.1242/dev.072157. [DOI] [PubMed] [Google Scholar]
- Reimer MM, Norris A, Ohnmacht J, Patani R, Zhong Z, Dias TB, et al. Dopamine from the brain promotes spinal motor neuron generation during development and adult regeneration. Dev Cell. 2013;25:478–491. doi: 10.1016/j.devcel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Muramatsu H, Ichihara-Tanaka K, Maeda N, Noda M, Yamamoto T, et al. Receptor-type protein tyrosine phosphatase zeta as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci Res. 2003;45:219–224. doi: 10.1016/s0168-0102(02)00226-2. [DOI] [PubMed] [Google Scholar]
- Sakakima H, Yoshida Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T. Midkine expression in rat spinal motor neurons following sciatic nerve injury. Brain Res Dev Brain Res. 2004;153:251–260. doi: 10.1016/j.devbrainres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Sakakima H, Yoshida Y, Yamazaki Y, Matsuda F, Ikutomo M, Ijiri K, et al. Disruption of the midkine gene (Mdk) delays degeneration and regeneration in injured peripheral nerve. J Neurosci Res. 2009;87:2908–2915. doi: 10.1002/jnr.22127. [DOI] [PubMed] [Google Scholar]
- Satoh J, Muramatsu H, Moretto G, Muramatsu T, Chang HJ, Kim ST, et al. Midkine that promotes survival of fetal human neurons is produced by fetal human astrocytes in culture. Brain Res Dev Brain Res. 1993;75:201–205. doi: 10.1016/0165-3806(93)90024-5. [DOI] [PubMed] [Google Scholar]
- Schafer M, Rembold M, Wittbrodt J, Schartl M, Winkler C. Medial floor plate formation in zebrafish consists of two phases and requires trunk-derived Midkine-a. Genes Dev. 2005;19:897–902. doi: 10.1101/gad.336305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277:35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- Tsutsui J, Uehara K, Kadomatsu K, Matsubara S, Muramatsu T. A new family of heparin-binding factors: strong conservation of midkine (MK) sequences between the human and the mouse. Biochem Biophys Res Commun. 1991;176:792–797. doi: 10.1016/s0006-291x(05)80255-4. [DOI] [PubMed] [Google Scholar]
- Unoki K, Ohba N, Arimura H, Muramatsu H, Muramatsu T. Rescue of photoreceptors from the damaging effects of constant light by midkine, a retinoic acid-responsive gene product. Invest Ophthalmol Vis Sci. 1994;35:4063–4068. [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Winkler C, Moon RT. Zebrafish mdk2, a novel secreted midkine, participates in posterior neurogenesis. Dev Biol. 2001;229:102–118. doi: 10.1006/dbio.2000.9967. [DOI] [PubMed] [Google Scholar]
- Winkler C, Schafer M, Duschl J, Schartl M, Volff JN. Functional divergence of two zebrafish midkine growth factors following fish-specific gene duplication. Genome Res. 2003;13(6A):1067–1081. doi: 10.1101/gr.1097503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Eriksson T, Vernersson E, Vigny M, Hallberg B, Palmer RH. The ligand Jelly Belly (Jeb) activates the Drosophila Alk RTK to drive PC12 cell differentiation, but is unable to activate the mouse ALK RTK. J Exp Zool B Mol Dev Evol. 2007;308:269–282. doi: 10.1002/jez.b.21146. [DOI] [PubMed] [Google Scholar]
- Yao S, Cheng M, Zhang Q, Wasik M, Kelsh R, Winkler C. Anaplastic lymphoma kinase is required for neurogenesis in the developing central nervous system of zebrafish. PLoS ONE. 2013;8:e63757. doi: 10.1371/journal.pone.0063757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Muramatsu T, Halfter W, Tsim KW, Peng HB. A role of midkine in the development of the neuromuscular junction. Mol Cell Neurosci. 1997;10:56–70. doi: 10.1006/mcne.1997.0638. [DOI] [PubMed] [Google Scholar]


