Fig. 6.
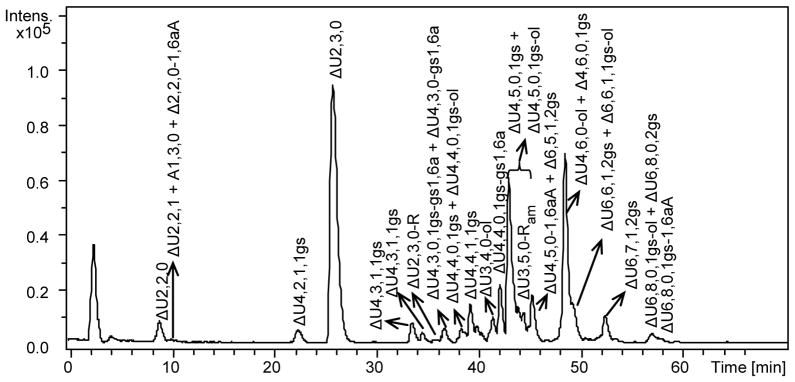
LC-MS chromatogram of the heparinase-digest of RO-enoxaparin
LC conditions: column C18 100 x 2.1 mm, 2.6 μm; eluents A and B – 10 mM DBA and 10 mM CH3COOH in H2O-CH3OH = 9:1 (v/v) and CH3OH, respectively; 0 min – 17% B, 10 min – 17%B, 30 – 42%, 50 min – 50% B, 65 min – 90%, 75 min – 90%B, 76 min – 17% B, 95 min – 17 %B; flow rate – 0.1 ml/min; injection volume – 5 μl.
Sodium borohydride reduction was used to distinguish between internal gs-unit and alditol end residue. For example, the double-charged ion with m/z 497.02 could correspond to both ΔU4,4,0,1gs and ΔU4,4,0-ol. After sodium borohydride reduction two peaks with m/z values were observed: a) m/z 498.02, indicating that this oligosaccharide containing one internal gs-unit (gsU), corresponds to the structure ΔUS-ANS6(S)-gsU-ANS6(S), where only one of the two sulfate groups within parenthesis is present; b) unmodified m/z 497.02, which should have an internal 2-O-sulfated uronic acid (US) and corresponds to ΔUS-ANS-US-A.olNS structure. The additional NaBH4 reduction of the RO-enoxaparin digest proved also the structure assigned to the minor tetrasaccharides terminating with an uronic acid remnant (ΔU4,4,1,1gs-R, m/z 577.03 for [M-2H]2− ion form, and ΔU4,5,0,1gs-R, m/z 596.01 for [M-2H]2− ion form), for which the m/z values remained constant after the additional reduction
