Summary
Recombinant human FVIIa (rhFVIIa) corrects the coagulopathy in hemophilia A and B as well as FVII deficiency. This is also the case in dogs until canine anti-human FVIIa antibodies develop (~2 weeks). Recombinant canine factor VIIa (rcFVIIa), successfully over-expressed by gene transfer in haemophilia dogs, has provided long-term haemostasis (>2 years). However, pharmacokinetics (PK), pharmacodynamics (PD) and safety of rcFVIIa after pharmacological administration have not been reported. We therefore wanted to explore the safety, PK and PD of rcFVIIa in dogs. A pilot study was set up to evaluate the safety as well as PK and PD of rcFVIIa after a single intravenous dose of 270 μg kg−1 to one HA and one haemostatically normal dog and to directly compare rcFVIIa with rhFVIIa in these two dogs. Single doses of rcFVIIa and rhFVIIa were well tolerated. No adverse events were observed. Pharmacokinetic characteristics including half-life (FVIIa activity: 1.2–1.8 h; FVIIa antigen 2.8–3.7 h) and clearance were comparable for rcFVIIa and rhFVIIa. Kaolin-activated thromboelastography approached normal in the HA dog with the improvement being most pronounced after rcFVIIa. This study provided the first evidence that administering rcFVIIa intravenously is feasible, safe, well tolerated and efficacious in correcting the haemophilic coagulopathy in canine HA and that rcFVIIa exhibits pharmacokinetic characteristics comparable to rhFVIIa in haemophilic and haemostatically competent dogs. This strengthens the hypothesis that rcFVIIa can be administered to dogs to mimic the administration of rhFVIIa to humans.
Keywords: animal model, haemophilia A, pharmacodynamics, pharmacokinetics, recombinant canine FVIIa, thromboelastography
Introduction
Recombinant human factor VIIa (rhFVIIa; NovoSeven ®; Novo Nordisk A/S, Bagsværd, Denmark) is effective in treating bleeding in haemophilia patients with inhibitors [1]. In normal physiology, FVIIa in combination with extra-vascular tissue factor (TF) exposed at a site of vascular injury, initiates coagulation [2,3]. When administered in pharmacologic doses, however, rhFVIIa acts as a FVIII or FIX bypassing agent by binding directly to activated platelets [4] and activating factor X (FX) on the activated platelet surface, thereby causing a thrombin burst and formation of a stable haemostatic plug [5] correcting the haemophilic phenotype.
Human FVIIa has been studied in haemostatically normal dogs [6], in haemophilic dogs [7] and in dogs with FVII deficiency [8]. These studies accurately predicted the safety, efficacy and relevant dosing directly applicable to the human clinic. Canine models continue to be important in the efforts to improve therapeutic options in haemophilia [9,10]. In support of the canine models, we have recently shown that rhFVIIa binds to canine TF essentially as to human TF [11] and that rhFVIIa binds to activated canine [12] and human [4] platelets with comparable characteristics. This substantiates the hypothesis that human FVIIa biology can be reliably recapitulated in canine models upon administration of rhFVIIa. However, administration of rhFVIIa to dogs causes formation of inhibitors to the xenoprotein and therefore studies are limited to a period of ~2 weeks in each dog. Intravenous administration of recombinant canine FVIIa (rcFVIIa) to haemophilic dogs would therefore be a valuable tool, e.g. to explore prophylactic FVIIa regimens [13] and to further elucidate the mechanism of action of FVIIa [5,14] and also as potential therapy in veterinary medicine.
The objectives of the study were to evaluate the pharmacokinetics (PK), pharmacodynamics (PD) and initial safety of rcFVIIa applied in a clinically relevant dose in a non-randomized cross-over pilot study using rhFVIIa as a direct comparator in one haemophilia A (HA) and one haemostatically normal dog.
Methods
Test compounds and in vitro characterization of their procoagulant potential
Recombinant cFVIIa [11] and rhFVIIa (NovoSeven®; Novo Nordisk A/S) were supplied in 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 150 mM NaCl, 10 mM CaCl2, pH 7.4 and 10 mM glycylglycine, 50 mM NaCl, 10 mM CaCl2, pH 6.6 respectively. Endotoxin content of the rcFVIIa preparation was 1.8 endotoxin units (EU) mg−1; accordingly, dosing at 270 μg kg−1 would expose the dogs to endotoxin well below the 100 EU kg−1 reported to induce a mild pyrogenic response in dogs [15].
Prior to infusions, the haemostatic potential of rcFVIIa was compared with rhFVIIa in spiking experiments. Briefly, to create samples with rcFVIIa/rhFVIIa content corresponding approximately to the maximal expected level after in vivo administration, rcFVIIa and rhFVIIa were diluted with buffer (15 mM HEPES, 150 mM NaCl, 5 mM CaCl2, pH 7.4) and spiked (2% v/v) in unstabilized blood from three HA and three haemostatically normal dogs. The spiked blood was then immediately tested in a kaolin-activated thromboelastography assay (kaolin-TEG) according to manufacturer (Haemoscope, Niles, IL, USA) instructions with duplicate measurements of each sample.
Study protocol
The study was conducted on the haemophilia dog colony at the Francis Owen Blood Research University of North Carolina, Chapel Hill, USA [10,16]. The protocol was approved by the Institutional Animal Care and Use Committee at the University of North Carolina. One HA dog and one haemostatically normal dog were included. Exclusion criteria were clinically overt illness (e.g. fever, bleeding, lameness, haematomas), abnormal clinical serum chemistry or haematology parameters, prior treatment with rhFVIIa and any medical treatment, including plasma transfusion, less than 14 days prior to inclusion.
Recombinant cFVIIa and rhFVIIa at 270 μg kg−1 were administered as a slow intravenous bolus over 120 s. Immediately prior to dosing, the injection volume was adjusted to 0.9 mL kg−1 by dilution of test compound with saline for injection (APP Pharmaceuticals, LLC, Schaumburg, IL, USA). Samplings were scheduled at 0 min (immediately prior to dosing) and at 5, 15 and 30 min and 1, 2, 3, 4, 6, 8, 12 and 24 h after dosing. Gentle cephalic venipuncture, employing a 21-gauge butterfly needle was used for infusion (one leg) and sampling (the other leg) with the animal under minimal manual restraint. To ensure optimal animal welfare throughout the entire sampling period, frequent physical examinations included auscultation of heart and lungs, observation of pulse, respiratory and heart rates and rectal body temperature. Dogs were allowed at least 1 week restitution between each dosing and rcFVIIa was administered first to avoid potential interference from anti-rhFVIIa antibodies [7].
Blood sampling and laboratory analyses
Samples for PK analysis were drawn into Stabilyte tubes (Biopool®Stabilyte™; Trinity Biotech, Bray, Ireland). Samples for kaolin-TEG were drawn at all time points except for 5 min and 3 and 6 h. Samples for haematology and coagulation profile were drawn into standard citrate prior to dosing and at 5 min and 1, 4, 8, 12 and 24 h after dosing. Samples for clinical chemistry were drawn into standard tubes for serum preparation immediately prior to dosing and 24 h after dosing. Blood for kaolin-TEG was drawn last in a 3 mL syringe without stabilizer applying gentle manual aspiration.
Factor VIIa in plasma was assessed by FVIIa clot activity (FVIIa:Clot) and antigen concentration (FVII:Ag) in species-specific assays. The human FVIIa:-Clot assay was performed as previously described [17]. A canine-specific FVIIa:Clot assay was developed by modifications of the human assay. Modifications included the use of canine FVII-deficient substrate plasma from beagle dogs homozygous for the FVII G96E mutation [18], canine soluble TF truncated after residue 217 (cTF1-217) [11] at 40 ng mL−1, and 0.05–2.0 ng mL−1 rcFVIIa as reference standard. FVII:Ag was measured in canine [19] and human (FVII EIA; Dako, Glostrup, Denmark) specific ELISAs, which measure total FVII:Ag (i.e. FVII as well as FVIIa antigen). Measurements were repeated in at least two separate analyses with duplicate measurements in each r2 > 0.99 was set as acceptance criteria for seven-point standard curves. Activity and antigen data were baseline corrected using predose FVIIa:Clot and FVII:Ag levels, respectively. To be able to directly compare rcFVIIa and rhFVIIa activity as well as antigen, all results were reported in ng mL−1.
For assessment of whole blood procoagulant potential, unstabilized samples were processed immediately (within 30 s) in the kaolin-TEG assay.
Coagulation profile
Activated partial thromboplastin time (APTT), prothrombin time (PT), d-dimer, fibrinogen, and antithrombin (AT) were completed at a specialized veterinary clinical pathology laboratory as previously described [20]. Thrombin–antithrombin (TAT) complexes were measured using a commercially available ELISA (Enzygnost TAT micro kit, Siemens Healthcare Diagnostics, Deerfield, IL, USA) as previously described [9,21].
Complete blood cell counts, including red blood cells (RBC), white blood cell (WBC) differentials and platelet count were assessed using an automated analyser (ABX Micros ABC Vet; HORIBA ABX, Irvine, CA, USA), calibrated for canine blood. Serum clinical chemistries were analysed in a specialized clinical pathology laboratory (Antech Diagnostics, Morrisville, NC, USA).
Safety assessment
The primary safety outcome was number of adverse events as assessed by physical examination (including careful inspection of the injection site) and complete blood counts (CBC) as well as plasma and serum analyses. Abnormal CBC data were defined as a more than 20% drop in platelet count or RBC, or a WBC more than 10% outside reference range. Fibrinogen and AT consumption resulting in measurements below reference range or d-dimer values above 1 μg mL−1 would define abnormal coagulation profile data. Any clinically relevant change in serum clinical chemistries postdose as compared with predose value would be perceived as abnormal.
Pharmacokinetic analysis
Pharmacokinetic parameters were accessed for the individual subjects by use of non-compartmental methods using WinNonlin (PC version 5.2; Pharsight, Cary, NC, USA). Briefly, the areas under the plasma FVIIa concentration vs. time curves (AUC) were calculated according to the trapezoidal rule. The half-life (t½) was estimated using observations obtained in samples taken 2–8 h after dosing. The infinite part of the curve was determined as Clast λ−1 where Clast is the last concentration and λ is the slope for the last phase. The peak concentration (Cmax) and time to reach the peak concentration (tmax) were read from the individual plasma concentration vs. time curves.
Pharmacodynamic analysis
Pharmacodynamic analysis was based on kaolin-TEG, TAT, PT and APTT measurements to evaluate the prohaemostatic potential of the test compounds. The kaolin-TEG analysis included evaluation of the reaction time (R), time to clot formation (K), angle (α) and maximum amplitude (MA). The R parameter is primarily related to plasma clotting factors and inhibitor activity; K represents kinetics of initial clot formation and is primarily related to clotting factors, fibrinogen and platelets; the angle represents the rate of fibrin formation and cross-linking and also depends on clotting factors, fibrinogen and platelets; MA is a direct function of fibrin–platelet interactions and represents the ultimate tensile strength of the fibrin clot [22]. Circulating TAT complex was taken as a measure of in vivo thrombin generation.
Results
Animals
For in vitro characterization of test compounds, blood was drawn from three HA dogs and three normal control dogs. The infusion study included one HA dog (ID: ‘M65’; gender: intact male; weight: 18 kg; age: 6 months) and one haemostatically normal dog (ID: ‘M48’; gender: intact female; weight: 17 kg; age: 10 months). Before sampling and dosing, the dogs were clinically examined without signs of disease and had normal complete blood count and clinical chemistry parameters. On the day of scheduled dosing of rhFVIIa to M65, 8 days after having completed dosing of rcFVIIa, M65 had a bleeding resulting in a ~150 mL subcutaneous haematoma in his left shoulder region due to fighting. Bleeding was treated by plasma transfusion and pain relief. Accordingly, dosing of rhFVIIa was postponed, until M65 again qualified for inclusion in the study.
In vitro characterization of test compounds by whole blood kaolin-TEG
Prior to infusion, rcFVIIa and rhFVIIa were spiked in unstabilized whole blood from HA (n = 3) and haemostatically normal control dogs (n = 3) at 0.5, 1.0 and 2.5 μg mL−1. The 2.5 μg mL−1 level was used to simulate a relevant rcFVIIa/rhFVIIa concentration in blood, shortly after a single dose of 270 μg kg−1. Kaolin-TEG parameters were normalized after spiking of 2.5 and 1.0 μg mL−1 rcFVIIa in whole blood from HA dogs. At 0.5 μg mL−1 rcFVIIa also improved kaolin-TEG parameters towards normal, although complete normalization, was not reached. Similarly, hrFVIIa improved all kaolin-TEG parameters of canine HA blood after spiking at 0.5, 1.0 and 2.5 μg mL−1, but none of these concentrations completely normalized the TEG profile. Dose dependency was observed for both compounds spiked in HA blood (Table 1). In comparison, neither rcFVIIa nor rhFVIIa markedly affected the kaolin-TEG profiles after spiking into blood from normal control dogs.
Table 1.
Preinfusion experiment. Effect of rcFVIIa and rhFVIIa spiked in canine haemophilia A blood assessed by kaolin-activated thromboelastography.
| Phenotype | Dose (μg mL−1) | Compound | R (min) | K (min) | α (°) | MA (mm) |
|---|---|---|---|---|---|---|
| Haemophilia A | – | Buffer | >60 | – | – | – |
| Haemophilia A | 2.5 | rcFVIIa | 3.8 ± 0.3 | 1.2 ± 0.1 | 73 ± 0.9 | 81 ± 1.1 |
| 1.25 | rcFVIIa | 6.8 ± 1.1 | 2.8 ± 0.4 | 60 ± 2.4 | 79 ± 1.4 | |
| 0.5 | rcFVIIa | 16 ± 1.7 | 5.3 ± 0.6 | 40 ± 4.3 | 78 ± 3.9 | |
| Haemophilia A | 2.5 | rhFVIIa | 31 ± 3.9 | 10 ± 4.9 | 26 ± 14 | 61 ± 15 |
| 1.25 | rhFVIIa | 39 ± 6.1 | 19 ± 12 | 18 ± 13 | 48 ± 19 | |
| 0.5 | rhFVIIa | 56 ± 17 | – | – | – | |
| Normal | – | Buffer | 5.3 ± 1.0 | 2.6 ± 1.2 | 56 ± 10 | 65 ± 5.5 |
| Normal | 2.5 | rcFVIIa | 2.2 ± 0.5 | 1.1 ± 0.2 | 73 ± 3.2 | 69 ± 3.4 |
| 1.25 | rcFVIIa | 2.9 ± 0.7 | 1.2 ± 0.2 | 71 ± 2.8 | 65 ± 6.0 | |
| 0.5 | rcFVIIa | 3.6 ± 0.7 | 1.6 ± 0.2 | 68 ± 3.1 | 68 ± 4.5 | |
| Normal | 2.5 | rhFVIIa | 6.1 ± 0.1 | 2.2 ± 0.3 | 61 ± 3.7 | 61 ± 4.4 |
| 1.25 | rhFVIIa | 5.8 ± 0.1 | 2.0 ± 0.3 | 61 ± 3.7 | 61 ± 4.4 | |
| 0.5 | rhFVIIa | 5.5 ± 0.6 | 1.8 ± 0.1 | 59 ± 7.4 | 65 ± 4.6 |
Data are shown as mean ± SD. Unstabilized blood from haemophilia A (n = 3) or haemostatically normal dogs (n = 3) was spiked with rcFVIIa or rhFVIIa as indicated and run in duplicates in kaolin-activated thromboelastography (kaolin-TEG). rcFVIIa, recombinant canine factor VIIa; rhFVIIa, recombinant human FVIIa (NovoSeven®); R, reaction time; K, clotting time; α, angle; MA, maximum amplitude; –, no value.
Safety
Both rcFVIIa and rhFVIIa were well tolerated and no adverse events were observed at any point. Both dogs were clinically well throughout dosing and sampling, and no injection site reactions were observed. Notably, no clinically relevant drop in platelet count, no evidence of AT or fibrinogen consumption was observed (Fig. 1). Haematology parameters stayed in the predose range throughout sampling periods, and 24 h serum clinical chemistries also stayed close to predose values (data not shown).
Fig. 1.
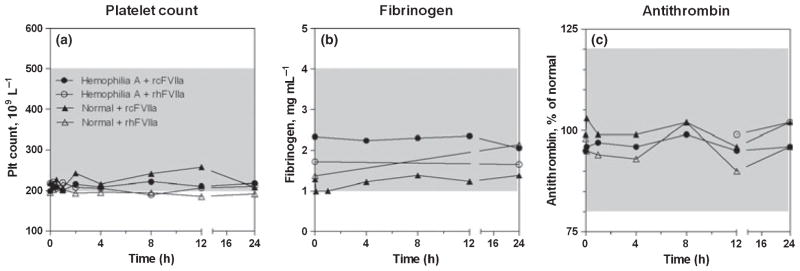
Coagulation was fully controlled after infusion in both dogs. No clinically relevant changes in platelet count, fibrinogen and antithrombin were observed after a single 270 μg kg−1 dose of recombinant canine factor VIIa and recombinant human FVIIa to one haemophilia A dog and one normal dog. Reference ranges are shown as shaded areas. Symbols and legends of (a) also apply to (b) and (c). Dogs from the Chapel Hill colony have been observed with platelet counts spanning ± ~20% of the depicted reference range. Hence, platelet counts in the lower end of the reference range as observed in these the two dogs is not an uncommon finding.
Pharmacokinetic profile
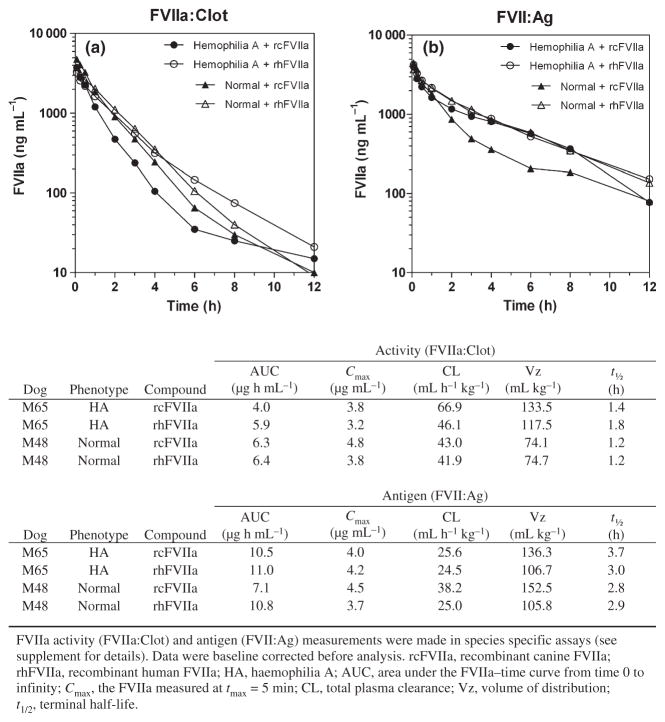
Pharmacokinetic profiles of rcFVIIa and rhFVIIa were comparable after intravenous dosing of both compounds at 270 μg kg−1 to one HA and one haemostatically normal dog (Fig. 2). Non-compartmental analysis of activity (i.e. FVIIa:Clot) and antigen (i.e. FVII:Ag) data yielded comparable PK parameters for rcFVIIa and rhFVIIa. Maximum exposure levels (Cmax) were measured 5 min after dosing (tmax = 5 min) and were comparable (3.2–4.8 μg mL−1) for rcFVIIa and rhFVIIa in both dogs. Although Cmax was comparable for rcFVIIa and rhFVIIa, irrespective of assay method, total drug exposure (AUC) was greater with calculations based on FVII:Ag as opposed to FVIIa:Clot data. Decreased activity vs. antigen exposure resulted from an increased clearance (CL) of FVIIa activity (42–67 mL h−1 kg−1) as compared with antigen CL (25– 38 mL h−1 kg−1). The difference in CL was also reflected in the terminal half-life, which was estimated to be 1.2–1.8 h and 2.8–3.7 h when based on activity and antigen measurements, respectively. In this study, baseline FVIIa:Clot activity ranged 1.1–2.5 ng mL−1 and baseline FVII:Ag ranged 815–1595 ng mL−1 when measured in the canine-specific assays.
Fig. 2.
Similar pharmacokinetic profiles of recombinant canine and human factor VIIa after a single 270 μg kg−1 dose to one haemophilia A and one normal dog. Profiles for (a) FVIIa activity (FVIIa:Clot) and (b) antigen (FVII:Ag) are shown.
Pharmacodynamic parameters
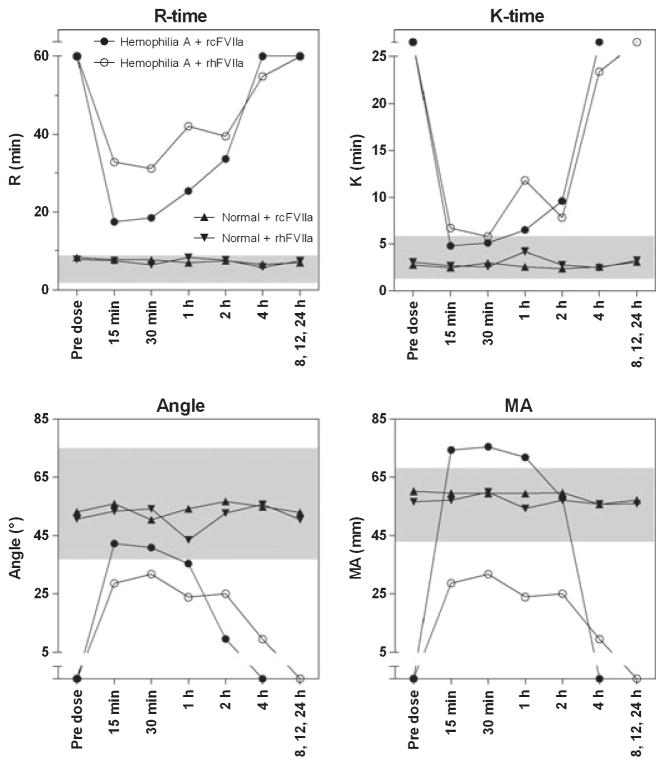
Kaolin-TEG parameters for the HA dog improved towards normal after dosing of rcFVIIa and rhFVIIa, with improvement being most pronounced after dosing of rcFVIIa, but with duration of improvement slightly longer for rhFVIIa (Fig. 3). In comparison, all kaolin-TEG parameters stayed within normal ranges [23] after dosing of rcFVIIa and rhFVIIa to the haemostatically normal dog. The in vivo pro-haemostatic effect of rcFVIIa and rhFVIIa was reflected in a rise in circulating TAT complex 5 min to 2 h after dosing. The presence of circulating TAT complexes was most pronounced with exposure of the haemostatically normal dog to rhFVIIa, as assessed by the area under the TAT time curve from 0 to12 h, with exposure of rcFVIIa leading to a more rapid increase, but also more rapid return to baseline (Fig. 4). Similarly, d-dimer levels increased slightly after infusion, again indicating controlled in vivo procoagulant activity of the compounds. Specifically, with rcFVIIa, d-dimer formation transiently increased above normal reference range, but below the defined safety threshold, in both dogs. In each dog the increase was observed at a single time point (HA, 8 h; normal, 12 h). Values had returned towards baseline values in both dogs at the 24 h time point. With rhFVIIa, d-dimer levels stayed within normal reference limits throughout the study, although a transient increase was also seen in the normal dog at 12 h. The pro-haemostatic effect of rcFVIIa and rhFVIIa was also reflected in standard coagulation profile parameters PT (data not shown).
Fig. 3.
Recombinant canine factor VIIa (rcFVIIa) and recombinant human FVIIa (rhFVIIa) were efficacious after infusion as assessed using thromboelastography. Change in kaolin-initiated thromboelastography (TEG) parameters after a single 270 μg kg−1 dose of rcFVIIa and rhFVIIa to one haemophilia A dog and one normal dog are shown as mean of duplicate measurements. TEG analysis was terminated if R-time was >60 min and therefore R-times reported as 60 min in this graph means ≥60 min. Similarly K-time, angle and MA values plotted outside the main y-axis mean ‘not measurable’. Symbols and legends as shown for R-time apply to all graphs in the figure.
Fig. 4.
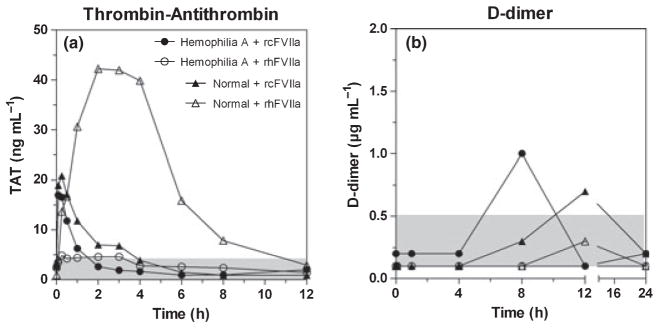
In vivo procoagulant potential as assessed by thrombin–antithrombin (TAT) complex and d-dimer formation after infusion. (a) Thrombin generation in vivo was as assessed by measuring circulating TAT complex. After infusion, a rise in TAT was observed for both compounds in both dogs, indicating that test compounds were haemostatically active. More TAT complex formed in the normal vs. in the haemophilia dog, probably reflecting the higher thrombin generation potential in the presence vs. absence of factor VIII. Faster FVIIa-AT complex formation, lower peak levels and faster return to baseline was observed with recombinant canine FVIIa (rcFVIIa) as compared with recombinant human FVIIa (rhFVIIa) in the normal dog. Normal reference range is indicated by the shaded area. (b) d-dimer formation transiently increased above normal reference range after rcFVIIa in both dogs. Values had returned towards baseline in both dogs at the 24 h time point. With rhFVIIa, d-dimer levels stayed within normal reference limits throughout the study, although a transient increase was also seen in the normal dog. Reference ranges are shown as shaded areas. Symbols and legends of (a) also apply to (b).
Discussion and conclusions
Recombinant cFVIIa was successfully produced in amounts needed for this pilot study demonstrating the safety, PK and PD of rcFVIIa after administration in a clinically relevant dose to both a healthy and a haemophilic dog. Clearly, the study would have been strengthened by inclusion of a larger number of dogs. However, we chose to administer a single 270 μg kg−1 dose to two dogs instead of 90 μg kg−1 doses to more dogs, because this dosing regimen is used in the human clinic and previous studies in dogs have yielded low inter-individual variation successfully modelling in the human setting even when small numbers of animals were included.
Recombinant cFVIIa was well tolerated by both dogs after single dose administration and no adverse events were observed. This is in line with the favourable safety profile observed with rhFVIIa in humans [24,25] and also with rhFVIIa in haemophilic dogs [7]. Platelet counts as well as plasma fibrinogen and AT levels were stable throughout the study and did not reveal any clinically relevant consumption. Although d-dimer stayed below the study threshold set to define clinically relevant increase, it reached a level slightly above the upper normal reference range at one time point in each dog after dosing of rcFVIIa. In comparison, a transient increase was seen in the normal, but not the HA dog after rhFVIIa. With rhFVIIa, the transient increase stayed within normal reference limits. In all cases, values had returned within normal limits and towards baseline in the 24-h sample.
Pharmacokinetics corresponded well to observations with rhFVIIa in humans [26,27] and to previous observations with rhFVIIa in HA and HB dogs [7]. Terminal plasma half-lives were shorter (1.2–1.8 h) when calculations were based on activity data, as compared with the longer half-life (2.8–3.7 h) obtained in calculations based on antigen. Complex formation between FVIIa and AT has been shown to occur both in vitro [28,29] and in vivo in humans [30]. Studies in dogs and mice indicate that FVIIa-AT complex formation also takes place after administration of rhFVIIa [31,32]. Hence, the different plasma FVIIa levels detected by the activity and antigen assays may, at least in part, be explained by the ability of the FVII:Ag, but not the FVIIa:Clot assay, to detect enzymatically inert FVIIa-AT complex. A previous study, assessing the survival and turnover of plasma derived canine FVII (pdcFVII) in dogs, identified activity in half-lives of 0.9–1.7 h (n = 6) after plasma transfusion and 1.1–4.1 h (n = 12) in cross-circulation experiments [33]. It should be noted that these experiments were carried out in FVII-deficient dogs [31], where PK parameters (e.g. Vz and CL) may be different from those observed in individuals with normal FVII levels [32]. However, in humans, rhFVIIa half-lives have not been observed to be markedly different in FVII deficient patients [34] as compared with patients with normal FVII levels [26,27]. Furthermore, it can not be ruled out that the relatively short activity half-lives of both rcFVIIa and rhFVIIa observed in this study could relate to the adolescent status of the two dogs; shorter activity half-lives (1.3–1.5 h [35]) and higher CL values have been observed for rhFVIIa in children with haemophilia as compared with the values reported for rhFVIIa in both adult normal healthy volunteers and in adults with haemophilia [35,36]. Thus, variation in PK parameters as observed in this study seems within limits of normal biological variation. As also observed with rhFVIIa in humans [26,27,35], both rcFVIIa and rhFVIIa were distributed into a volume (Vz) corresponding to ~150–300% of total plasma volume, irrespective of assay method. In this study, baseline FVIIa:Clot activity in canine plasma ranged 1.1–2.5 ng mL−1 (corresponding to ~0.2% of total FVII:Ag). Such levels are comparable to those measured in human plasma using the human-specific FVIIa:Clot assay [17]. Conversely, the levels found in the present study stand in some contrast to the more than 100 times higher FVIIa:Clot activity previously reported after measurements of normal canine plasma in the human assay [9]. This difference is probably explained by the different assays used, and confirms that it is important to address species specificity by optimized assay conditions.
Recombinant human FVIIa has previously been shown efficacious in treating canine haemophilic bleeds [7]. Thus, to evaluate the potential efficacy of rcFVIIa, we compared rcFVIIa with rhFVIIa in a kaolin-TEG assay [37,38]. Because of the differential binding of rcFVIIa and rhFVIIa to human TF (hTF), hTF-initiated TEG would potentially have biased the results in favour of rhFVIIa [11]. A marked improvement of the kaolin-TEG coagulation profile of the HA dog was observed with both compounds; the initial improvement was most pronounced with rcFVIIa (R-time: 18 min vs. 33 min for rcFVIIa vs. rhFVIIa), whereas the duration of action was marginally prolonged with rhFVIIa as compared with rcFVIIa. However, these differences could reflect normal biological variation and should be explored in more dogs before final conclusions are drawn. Overall, postinfusion kaolin-TEG measurements corroborated data obtained with spiking of rcFVIIa and rhFVIIa in HA blood prior to infusion. However, as compared with spiking experiments, markedly higher plasma concentrations of both rcFVIIa and rhFVIIa were needed in vivo to partially normalize the ex vivo kaolin-TEG profile of canine HA blood. This finding confirms that infusion studies are needed to fully appreciate the complex interplay of rcFVIIa with other components of the haemostatic system, e.g. cellular and soluble plasma components (such as AT) as well as the surrounding vasculature.
The high platelet dependent activity of rcFVIIa was reflected in the near normalization of kaolin-TEG parameters in this study. As expected, FVIIa activity was also reflected by supraphysiologic reduction in PT after dosing. However, as previously shown [11], data from the PT assay indicated that human TF was a poor stimulator of rcFVIIa activity, potentially misleading the unaware interpreter to a wrongful conclusion of increased potency of rhFVIIa over rcFVIIa. In addition, a transient decrease in APTT and the increase in TAT and d-dimer after administration of both compounds indicated in vivo activation of the coagulation system.
In the haemostatically normal dog, TAT complexes rose to a peak around 40 ng mL−1, 4 h postinfusion, and declined almost back to baseline, 8 hours after infusion of rhFVIIa. In humans, a similar profile was seen in a clinical trial with dosing of rhFVIIa to haemostatically normal men undergoing retropubic prostatectomy [39]. In comparison, TAT levels increased only slightly above baseline after dosing of rhFVIIa to the HA dog. Conversely, after dosing of rcFVIIa, TAT levels quickly raised to peaks at around 20 ng mL−1, half of that observed with rhFVIIa in the normal dog. Furthermore, after dosing of rcFVIIa, TAT formation displayed faster kinetics, e.g. peak levels reached 5–30 min after infusion, in both dogs. Notably, continuous expression of cFVIIa in HA and HB dogs following successful gene transfer was accompanied by fluctuations of d-dimer and TAT in a similar range but over long periods [9]. The variation in TAT profiles was not addressed specifically in this study, and would require additional confirmation in a larger number of dogs. No clinically relevant changes in, and thereby no consumption of platelets, fibrinogen and AT were observed, indicating that the coagulation was fully controlled by physiological mechanisms both in the HA and the haemostatically normal dog postinfusion.
The potential for FVIIa gene therapy for treating haemophilia patients has been studied in haemophilia dogs [9] after initial proof of concept was obtained in mice [40]. In both species, the bleeding phenotype was corrected, albeit cardiac and pulmonary pathology was observed in mice [41], but not with rcFVIIa in dogs to date. However, as gene therapy is only clinically applicable at present in controlled clinical trials, it seems pertinent to explore safety and efficacy of the prophylactic use of pharmacologically administered rhFVIIa in a relevant animal model. In this setting, rcFVIIa dosed to haemophilia dogs offers several advantages over pharmacological application of rmFVIIa in mouse models. Haemophilic dogs exhibit a disease phenotype closely recapitulating that of human haemophilia, dogs are more comparable with humans with regard to bodyweight as well as FVIIa dosing requirement, and binding of rcFVIIa and rhFVIIa to canine TF is comparable to the binding of rhFVIIa to human TF [11].
Altogether, the findings of this pilot study are in line with previous reports exploring plasma-derived canine FVII [33] as well as rhFVIIa [7] and rcFVIIa [9] in dogs, and similar to observations from human clinical trials with rhFVIIa [26,27,35,39,42]. Thereby, this study corroborates the accurate recapitulation of human biology by canine models within this particular area of haemostasis. The fact that highly valuable data on FVIIa have previously been obtained in studies with a single or few dogs [6–9] and that PK parameters were comparable to what has previously been observed with plasma-derived canine FVII and rhFVIIa in both canine and human studies, indicates that data from this pilot study may be of significant value for enhanced interpretation of data obtained in canine haemophilia models. It would be interesting to study in more details the PK and PD of rcFVIIa in additional haemophilic dogs as well as in dogs with congenital FVII deficiency [34]. Similarly, it would be interesting to compare efficacy of replacement [43] and bypassing haemophilia treatment regimens in haemophilic dogs. Furthermore, the availability of rcFVIIa would potentially allow treatment studies in larger cohorts of dogs with congenital as well as acquired bleeding disorders.
In conclusion, this study provides the first evidence that administering rcFVIIa intravenously is feasible, safe, well tolerated and efficacious in correcting the haemophilic coagulopathy in canine HA, and exhibits pharmacokinetic characteristics comparable to rhFVIIa in haemophilic and haemostatically competent dogs. This strengthens the hypothesis that rcFVIIa can be administered to dogs to mimic the administration of rhFVIIa to humans.
Acknowledgments
The authors gratefully acknowledge participating staff at the Department of Small Animal Clinical Sciences, Faculty of Life Sciences, University of Copenhagen, Denmark, at Francis Owen Blood Research Laboratory, University of North Carolina (UNC), Chapel Hill, USA and at Novo Nordisk A/S, Denmark. The study was financially supported through an unrestricted grant to T.K. by The Faculty of Life Sciences, University of Copenhagen, The Danish Ministry of Science, Technology and Innovation, and Novo Nordisk A/S, Denmark. The colony of dogs at UNC was supported by NIH/NHLBI R24HL063098-12.
Footnotes
Disclosures
HA, MK, ME, MT and TK are all employees of Novo Nordisk A/S.
References
- 1.Abshire T, Kenet G. Recombinant factor VIIa: review of efficacy, dosing regimens and safety in patients with congenital and acquired factor VIII or IX inhibitors. J Thromb Haemost. 2004;2:899–909. doi: 10.1111/j.1538-7836.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- 2.Rapaport SI, Rao LV. The tissue factor pathway: how it has become a ‘prima ballerina’. Thromb Haemost. 1995;74:7–17. [PubMed] [Google Scholar]
- 3.Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol. 2006;26:41–8. doi: 10.1161/01.ATV.0000193624.28251.83. [DOI] [PubMed] [Google Scholar]
- 4.Kjalke M, Kjellev S, Rojkjaer R. Preferential localization of recombinant factor VIIa to platelets activated with a combination of thrombin and a glycoprotein VI receptor agonist. J Thromb Haemost. 2007;5:774–80. doi: 10.1111/j.1538-7836.2007.02389.x. [DOI] [PubMed] [Google Scholar]
- 5.Monroe DM, Hoffman M, Oliver JA, Roberts HR. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99:542–7. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 6.Hedner U, Kisiel W. Use of human factor VIIa in the treatment of two hemophilia A patients with high-titer inhibitors. J Clin Invest. 1983;71:1836–41. doi: 10.1172/JCI110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkhous KM, Hedner U, Garris JB, Diness V, Read MS. Effect of recombinant factor VIIa on the hemostatic defect in dogs with hemophilia A, hemophilia B, and von Willebrand disease. Prroc Natl Acad Sci USA. 1989;86:1382–6. doi: 10.1073/pnas.86.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Withnall E, Giger U. Effects of recombinant human activated factor VII and canine fresh frozen plasma in Beagles with hereditary coagulation factor VII deficiency (abstract) J Vet Int Med. 2006;20:766. [Google Scholar]
- 9.Margaritis P, Roy E, Aljamali MN, et al. Successful treatment of canine hemophilia by continuous expression of canine FVIIa. Blood. 2009;113:3682–9. doi: 10.1182/blood-2008-07-168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols TC, Dillow AM, Franck HW, et al. Protein replacement therapy and gene transfer in canine models of hemophilia A, hemophilia B, von willebrand disease, and factor VII deficiency. ILAR J. 2009;50:144–67. doi: 10.1093/ilar.50.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudsen T, Kristensen AT, Sorensen BB, Olsen OH, Stennicke HR, Petersen LC. Characterization of canine coagulation factor VII and its complex formation with tissue factor: canine-human cross-species compatibility. J Thromb Haemost. 2010;8:1763–72. doi: 10.1111/j.1538-7836.2010.03931.x. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen T, Kjalke M, Tranholm M, Nichols TC, Jensen AL, Kristensen AT. Detection of canine coated platelets and their rhFVIIa binding by flow cytometry. Am J Vet Res. doi: 10.2460/ajvr.72.8.1007. in press. [DOI] [PubMed] [Google Scholar]
- 13.Konkle BA, Ebbesen LS, Erhardtsen E, et al. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost. 2007;5:1904–13. doi: 10.1111/j.1538-7836.2007.02663.x. [DOI] [PubMed] [Google Scholar]
- 14.Gopalakrishnan R, Hedner U, Ghosh S, Nayak R, Allen TC, Pendurthi UR, Rao LV. Bio-distribution of pharmacologically administered rFVIIa. J Thromb Haemost. 2010;8(2):301–10. doi: 10.1111/j.1538-7836.2009.03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeMay DR, LeMay LG, Kluger MJ, D’Alecy LG. Plasma profiles of IL-6 and TNF with fever-inducing doses of lipopolysaccharide in dogs. Am J Physiol. 1990;259:R126–32. doi: 10.1152/ajpregu.1990.259.1.R126. [DOI] [PubMed] [Google Scholar]
- 16.Graham JB, Buckwalter JA, Hartley LJ, Brinkhous KM. Canine Hemophilia: observations on the course, the clotting anomaly, and the effect of blood transfusions. J Exp Med. 1949;90:97–111. doi: 10.1084/jem.90.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81:734–44. [PubMed] [Google Scholar]
- 18.Callan MB, Aljamali MN, Margaritis P, et al. A novel missense mutation responsible for factor VII deficiency in research Beagle colonies. J Thromb Haemost. 2006;4:2616–22. doi: 10.1111/j.1538-7836.2006.02203.x. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen T, Kjelgaard-Hansen M, Tranholm M, et al. Canine specific ELISA for coagulation factor VII. [Accessed April 11, 2011.];Vet J. 2011 doi: 10.1016/j.tvjl.2010.11.010. http://www.ncbi.nlm.nih.gov/pubmed/21216638. [DOI] [PMC free article] [PubMed]
- 20.Wiinberg B, Jensen AL, Rozanski E, et al. Tissue factor activated thromboelastography correlates to clinical signs of bleeding in dogs. Vet J. 2009;179:121–9. doi: 10.1016/j.tvjl.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Ravanat C, Freund M, Dol F, et al. Cross-reactivity of human molecular markers for detection of prethrombotic states in various animal species. Blood Coagul Fibrinolysis. 1995;6:446–55. doi: 10.1097/00001721-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Mallett SV, Cox DJ. Thrombelastography. Br J Anaesth. 1992;69:307–13. doi: 10.1093/bja/69.3.307. [DOI] [PubMed] [Google Scholar]
- 23.Bauer N, Eralp O, Moritz A. Establishment of reference intervals for kaolin-activated thromboelastography in dogs including an assessment of the effects of sex and anticoagulant use. J Vet Diagn Invest. 2009;21:641–8. doi: 10.1177/104063870902100508. [DOI] [PubMed] [Google Scholar]
- 24.Roberts HR, Monroe DM, III, Hoffman M. Safety profile of recombinant factor VIIa. Semin Hematol. 2004;41(Suppl 1):101–8. doi: 10.1053/j.seminhematol.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Levi M, Peters M, Buller HR. Efficacy and safety of recombinant factor VIIa for treatment of severe bleeding: a systematic review. Crit Care Med. 2005;33:883–90. doi: 10.1097/01.ccm.0000159087.85970.38. [DOI] [PubMed] [Google Scholar]
- 26.Lindley CM, Sawyer WT, Macik BG, et al. Pharmacokinetics and pharmacodynamics of recombinant factor VIIa. Clin Pharmacol Ther. 1994;55:638–48. doi: 10.1038/clpt.1994.80. [DOI] [PubMed] [Google Scholar]
- 27.Fridberg MJ, Hedner U, Roberts HR, Erhardtsen E. A study of the pharmacokinetics and safety of recombinant activated factor VII in healthy Caucasian and Japanese subjects. Blood Coagul Fibrinolysis. 2005;16:259–66. doi: 10.1097/01.mbc.0000169218.15926.34. [DOI] [PubMed] [Google Scholar]
- 28.Lawson JH, Butenas S, Ribarik N, Mann KG. Complex-dependent inhibition of factor VIIa by antithrombin III and heparin. J Biol Chem. 1993;268:767–70. [PubMed] [Google Scholar]
- 29.Rao LV, Nordfang O, Hoang AD, Pendurthi UR. Mechanism of antithrombin III inhibition of factor VIIa/tissue factor activity on cell surfaces. Comparison with tissue factor pathway inhibitor/factor Xa-induced inhibition of factor VIIa/tissue factor activity. Blood. 1995;85:121–9. [PubMed] [Google Scholar]
- 30.Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–62. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 31.Petersen LC, Elm T, Ezban M, et al. Plasma elimination kinetics for factor VII are independent of its activation to factor VIIa and complex formation with plasma inhibitors. Thromb Haemost. 2009;101:818–26. [PubMed] [Google Scholar]
- 32.Petersen LC, Karpf DM, Agerso H, et al. Intravascular inhibition of factor VIIa and the analogue NN1731 by antithrombin. Br J Haematol. 2011;152:99–107. doi: 10.1111/j.1365-2141.2010.08432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodds WJ, Packham MA, Rowsell HC, Mustard JF. Factor VII survival and turnover in dogs. Am J Physiol. 1967;213:36–42. doi: 10.1152/ajplegacy.1967.213.1.36. [DOI] [PubMed] [Google Scholar]
- 34.Berrettini M, Mariani G, Schiavoni M, et al. Pharmacokinetic evaluation of recombinant, activated factor VII in patients with inherited factor VII deficiency. Haematologica. 2001;86:640–5. [PubMed] [Google Scholar]
- 35.Villar A, Aronis S, Morfini M, et al. Pharmacokinetics of activated recombinant coagulation factor VII (NovoSeven) in children vs. adults with haemophilia A. Haemophilia. 2004;10:352–9. doi: 10.1111/j.1365-2516.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 36.Erhardtsen E. Pharmacokinetics of recombinant activated factor VII (rFVIIa) Semin Thromb Hemost. 2000;26:385–91. doi: 10.1055/s-2000-8457. [DOI] [PubMed] [Google Scholar]
- 37.Young G, Ebbesen LS, Viuff D, et al. Evaluation of thromboelastography for monitoring recombinant activated factor VII ex vivo in haemophilia A and B patients with inhibitors: a multicentre trial. Blood Coagul Fibrinolysis. 2008;19:276–82. doi: 10.1097/MBC.0b013e3283001cdc. [DOI] [PubMed] [Google Scholar]
- 38.Young G, Zhang R, Miller R, Yassin D, Nugent DJ. Comparison of kaolin and tissue factor activated thromboelastography in haemophilia. Haemophilia. 2010;16:518–24. doi: 10.1111/j.1365-2516.2009.02165.x. [DOI] [PubMed] [Google Scholar]
- 39.Friederich PW, Henny CP, Messelink EJ, et al. Effect of recombinant activated factor VII on perioperative blood loss in patients undergoing retropubic prostatectomy: a double-blind placebo-controlled randomised trial. Lancet. 2003;361:201–5. doi: 10.1016/S0140-6736(03)12268-4. [DOI] [PubMed] [Google Scholar]
- 40.Margaritis P, Arruda VR, Aljamali M, Camire RM, Schlachterman A, High KA. Novel therapeutic approach for hemophilia using gene delivery of an engineered secreted activated Factor VII. J Clin Invest. 2004;113:1025–31. doi: 10.1172/JCI20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aljamali MN, Margaritis P, Schlachterman A, et al. Long-term expression of murine activated factor VII is safe, but elevated levels cause premature mortality. J Clin Invest. 2008;118:1825–34. doi: 10.1172/JCI32878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macik BG, Lindley CM, Lusher J, et al. Safety and initial clinical efficacy of three dose levels of recombinant activated factor VII (rFVIIa): results of a phase I study. Blood Coagul Fibrinolysis. 1993;4:521–7. doi: 10.1097/00001721-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Sabatino DE, Furlan FC, Toso R, et al. Recombinant canine B-domain deleted FVIII exhibits high specific activity and is safe in the canine hemophilia A model. Blood. 2009;114:4562–5. doi: 10.1182/blood-2009-05-220327. [DOI] [PMC free article] [PubMed] [Google Scholar]