Abstract
Nature’s diversity is one of the biggest resources of therapeutic lead compounds. Traditionally-used herbal remedies harbor a variety of bioactive compounds providing researchers with starting points for drug development. Ethnopharmacological investigations of uterotonic plant preparations identified a class of circular and disulfide-rich peptides, called cyclotides, to exhibit strong uterine contractions. In humans one of the physiological regulators of the myometrial contractility is the nonapeptide oxytocin acting on its cognate G protein-coupled receptors. They are considered to represent one of the most promising drug targets with ~30% of all currently marketed drugs acting on these transmembrane receptors. Based on observed similarities regarding the activity and structure of plant cyclotides with human oxytocin we analyzed the pharmacological principle of their action and identified the oxytocin and vasopressin 1a receptors as molecular targets of cyclotides from the Rubiaceae plant Oldenlandia affinis. Using a synthetic approach, the sequence of the native cyclotide kalata B7 was used to design oxytocin-like nonapeptides with nanomolar affinity and selectivity for the oxytocin receptor. This provides formal proof for the use of cyclotides as templates in peptide ligand design. At a more general level, mining of naturally-occurring peptides is a promising tool for the identification and the design of novel G protein-coupled receptor ligands.
Keywords: cyclotides, oxytocin, G protein-coupled receptors, peptide, plant, ligand, selectivity
Traditional medicine strongly relies on beneficial effects of herbal remedies. The bioactive constituents of such plants display a rich source for the discovery of novel compounds with potential for pharmacological applications and drug development. Besides small organic molecules, i.e. secondary metabolites, plants express a variety of gene-encoded peptides. One particular class of naturally-occurring peptides that has been identified are the so-called cyclotides. These mini-proteins comprise a unique structural topology, i.e. the combination of 3 interlocked disulfide-bonds and a N-to-C cyclized backbone.1 Their distribution in the plant kingdom is still under investigation, but cyclotides have been isolated from species of the Rubiaceae (coffee-), Violaceae (violet-), Cucurbitaceae (squash-), Fabaceae (pea-), Solanaceae (potato-), and Poaceae (grass-) families (summarized in Koehbach et al.2). Their estimated number of more than 50,000 within the coffee family alone makes them one of the largest class of plant compounds, and hence a rich source for the discovery of bioactive peptides.3,4 A range of intrinsic bioactivities has been reported for cyclotides (summarized in Thell, Hellinger et al.5), yet their underlying molecular mechanism, in particular a specific target receptor for cyclotides, has not been discovered hitherto. First evidence for a direct target modulation of cyclotides came from reports of their uterotonic activity6,7 and their action as neurotensin antagonists.8 Ethnopharmacological investigations performed by Lorents Gran in the 1970s on herbal remedies used in traditional Congolese medicine identified aqueous extracts of the plant Oldenlandia affinis (Fig. 1) – known to indigenous people as “kalata-kalata” – to elicit strong uterine contractions in rats and rabbits, as well as on isolated human uterine strips.9,10 Several uterotonic signaling pathways have been described previously and one important physiological regulator of uterine contraction is the neuropeptide oxytocin.11 In humans, oxytocin and the closely related vasopressin, act on 4 cognate receptors, i.e., the oxytocin and 3 vasopressin (1a, 1b, and 2) receptors. These receptors belong to the family of G protein-coupled receptors (GPCR). GPCRs are promising drug targets since > 30% of the currently marketed drugs elicit their actions by binding to these transmembrane receptors. However, only ∼10% of all GPCRs are targeted by approved drugs. Hence it was intriguing to pharmacologically investigate the uterotonic effects of cyclotides to elucidate their molecular target(s).

Figure 1. Flower of Oldenlandia affinis, a traditional medicinal plant used to speed up labor. Koehbach et al.12 characterized the molecular principle behind this uterotonic activity and isolated the first oxytocic cyclotide that has been used as template for peptide GPCR ligand design. Photograph by courtesy of David Wilson (James Cook University, Australia). Background: cartoon of kalata B7 structure (PDB ID: 2M9O).
In our study we followed a bioactivity- and pharmacology-guided fractionation approach and monitored the activity of a cyclotide extract, semipure cyclotide fractions as well as isolated cyclotides by measuring their ability to contract human uterus cells, and bind to or activate cells heterologously expressing the human oxytocin- or vasopressin 1a receptors. This led to the identification of the cyclotide kalata B7 as partial agonist of these 2 receptors.12 The “active cyclotide” was structurally characterized by NMR spectroscopy and the cyclotide-receptor interaction was investigated using a human oxytocin receptor homology model13 in comparison to the published ligand-bound structure of the neurotensin receptor.14 In the crystal structure the neurotensin peptide NTS8–13 is confined to the extracellular surface and does not enter deep into the hydrophobic core of the receptor. This is in agreement with the structural alignment model of the cyclotide-oxytocin receptor complex: the majority of the cyclotide connects to the extracellular loops of the receptor and extends the lid over the binding pocket15 and only loop 3 of kalata B7 is buried within the ligand binding pocket of the receptor (Fig. 2). We are aware of the limitations of homology models, though it indicates that despite the larger size of cyclotides in comparison to endogenous neuropeptide ligands, cyclotides are capable of penetrating the ligand-binding core of peptide GPCRs. This has been confirmed by radioligand displacement and second messenger-based reporter experiments of native and modified cyclotides with the human oxytocin- and vasopressin V1a receptors.12
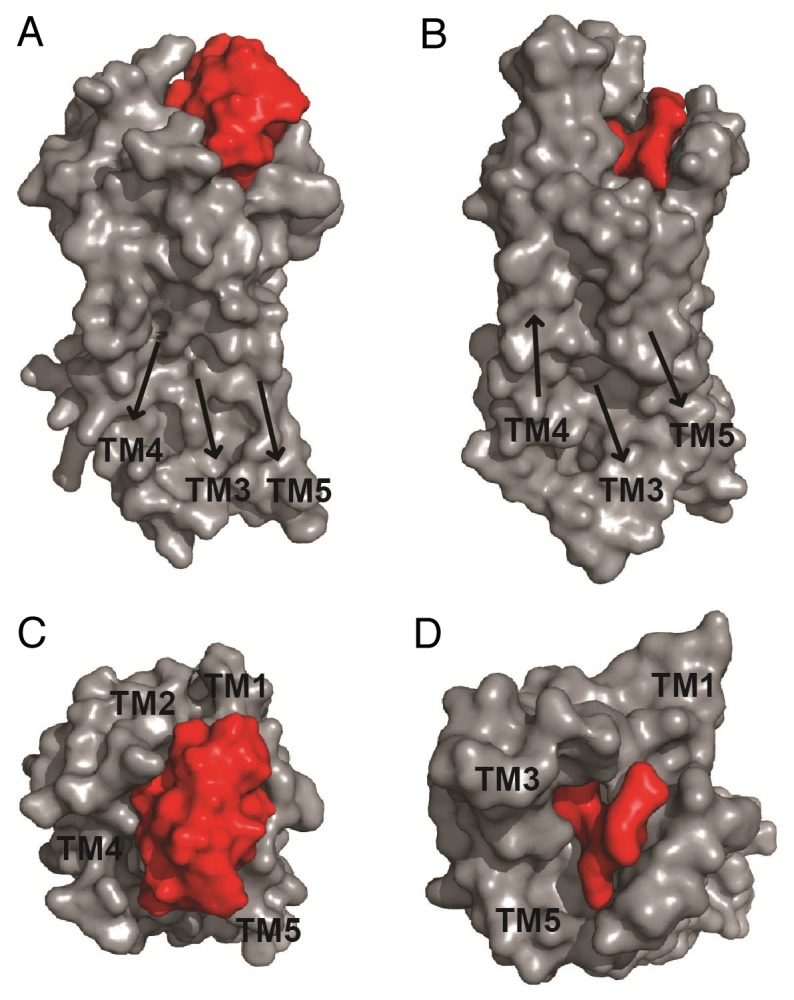
Figure 2. Comparison of ligand-bound oxytocin- and neurotensin receptors. The NMR structure of kalata B7 (PDB ID: 2M9O) was fitted into the cavity of a model of the human oxytocin receptor (OTR) based on the mouse µ-opioid crystal structure (A, C).13 The structure of the ligand-bound rat neurotensin receptor (NTSR, PDB ID: 4GRV) receptor is shown for comparison (B, D). Surface representations of both receptors are shown from (A, B) side view and (C, D) top view. Peptide and receptor are colored in red and gray, respectively. Arrows indicate orientations of transmembrane domains (TM). Images were prepared using PyMOL.
Based on these findings, we designed several oxytocin-like nonapeptides using the sequence of the native oxytocic cyclotide kalata B7 as a template. The peptide [G5,T7,S9]-oxytocin (kB7-OT1) turned out to be a potent and selective agonist of the human oxytocin receptor. This is of great interest from a drug design point-of-view, since oxytocin, vasopressin, and many analogs synthesized to date have been lacking receptor selectivity.16,17 Our findings thus highlight the potential of exploiting cyclotides as templates for the design of peptide GPCR ligands. Cyclotides are an abundant class of plant peptides and recent approaches combining peptidomics with transcriptomics analysis are very encouraging to increase the discovery rate of novel cyclotides.2 Given the diversity of plant cyclotides as well as the overall number of GPCRs (the human genome encodes for at least 800 different GPCRs18) that display promising drug targets, our results provide the proof-of-principle for the development of cyclotide-based peptide ligands using a combination of 2 disciplines, i.e., ethnopharmacology and chemical biology. However, at least one question remains unanswered: what is the evolutionary role and physiological function of cyclotides? Knowing that cyclotides modulate peptide GPCRs, one may speculate that their biological target are insect-, or more generally invertebrate neuropeptide receptors to affect the development, reproductive behavior, or water homeostasis of these animals,19 for the purpose of defense or communication.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/27583
References
- 1.Craik DJ, Daly NL, Bond T, Waine C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol. 1999;294:1327–36. doi: 10.1006/jmbi.1999.3383. [DOI] [PubMed] [Google Scholar]
- 2.Koehbach J, Attah AF, Berger A, Hellinger R, Kutchan TM, Carpenter EJ, Rolf M, Sonibare MA, Moody JO, Wong GK, et al. Cyclotide discovery in Gentianales revisited-identification and characterization of cyclic cystine-knot peptides and their phylogenetic distribution in Rubiaceae plants. Biopolymers. 2013;100:438–52. doi: 10.1002/bip.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruber CW, Elliott AG, Ireland DC, Delprete PG, Dessein S, Göransson U, Trabi M, Wang CK, Kinghorn AB, Robbrecht E, et al. Distribution and evolution of circular miniproteins in flowering plants. Plant Cell. 2008;20:2471–83. doi: 10.1105/tpc.108.062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber CW. Global cyclotide adventure: a journey dedicated to the discovery of circular peptides from flowering plants. Biopolymers. 2010;94:565–72. doi: 10.1002/bip.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thell K, Hellinger R, Schabbauer G, Gruber CW. Immunosuppressive peptides and their therapeutic applications. Drug Discov Today. 2013 doi: 10.1016/j.drudis.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gran L. An oxytocic principle found in Oldenlandia affinis DC. Medd Nor Farm Selsk. 1970;12:173–80. [Google Scholar]
- 7.Gran L. Oxytocic principles of Oldenlandia affinis. Lloydia. 1973;36:174–8. [PubMed] [Google Scholar]
- 8.Witherup KM, Bogusky MJ, Anderson PS, Ramjit H, Ransom RW, Wood T, Sardana M. Cyclopsychotride A, a biologically active, 31-residue cyclic peptide isolated from Psychotria longipes. J Nat Prod. 1994;57:1619–25. doi: 10.1021/np50114a002. [DOI] [PubMed] [Google Scholar]
- 9.Gran L. On the effect of a polypeptide isolated from “Kalata-Kalata” (Oldenlandia affinis DC) on the oestrogen dominated uterus. Acta Pharmacol Toxicol (Copenh) 1973;33:400–8. doi: 10.1111/j.1600-0773.1973.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 10.Gran L, Sandberg F, Sletten K. Oldenlandia affinis (R&S) DC. A plant containing uteroactive peptides used in African traditional medicine. J Ethnopharmacol. 2000;70:197–203. doi: 10.1016/S0378-8741(99)00175-0. [DOI] [PubMed] [Google Scholar]
- 11.Gruber CW, O’Brien M. Uterotonic plants and their bioactive constituents. Planta Med. 2011;77:207–20. doi: 10.1055/s-0030-1250317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koehbach J, O’Brien M, Muttenthaler M, Miazzo M, Akcan M, Elliott AG, Daly NL, Harvey PJ, Arrowsmith S, Gunasekera S, et al. Oxytocic plant cyclotides as templates for peptide G protein-coupled receptor ligand design. Proc Natl Acad Sci U S A. 2013;110:21183–8. doi: 10.1073/pnas.1311183110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehbach J, Stockner T, Bergmayr C, Muttenthaler M, Gruber CW. Insights into the molecular evolution of oxytocin receptor ligand binding. Biochem Soc Trans. 2013;41:197–204. doi: 10.1042/BST20120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, et al. Structure of the agonist-bound neurotensin receptor. Nature. 2012;490:508–13. doi: 10.1038/nature11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheatley M, Wootten D, Conner MT, Simms J, Kendrick R, Logan RT, Poyner DR, Barwell J. Lifting the lid on GPCRs: the role of extracellular loops. Br J Pharmacol. 2012;165:1688–703. doi: 10.1111/j.1476-5381.2011.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–28. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber CW, Koehbach J, Muttenthaler M. Exploring bioactive peptides from natural sources for oxytocin and vasopressin drug discovery. Future Med Chem. 2012;4:1791–8. doi: 10.4155/fmc.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber CW, Muttenthaler M, Freissmuth M. Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors. Curr Pharm Des. 2010;16:3071–88. doi: 10.2174/138161210793292474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber CW. Physiology of invertebrate oxytocin and vasopressin neuropeptides. Exp Physiol. 2013;••• doi: 10.1113/expphysiol.2013.072561. [DOI] [PMC free article] [PubMed] [Google Scholar]


