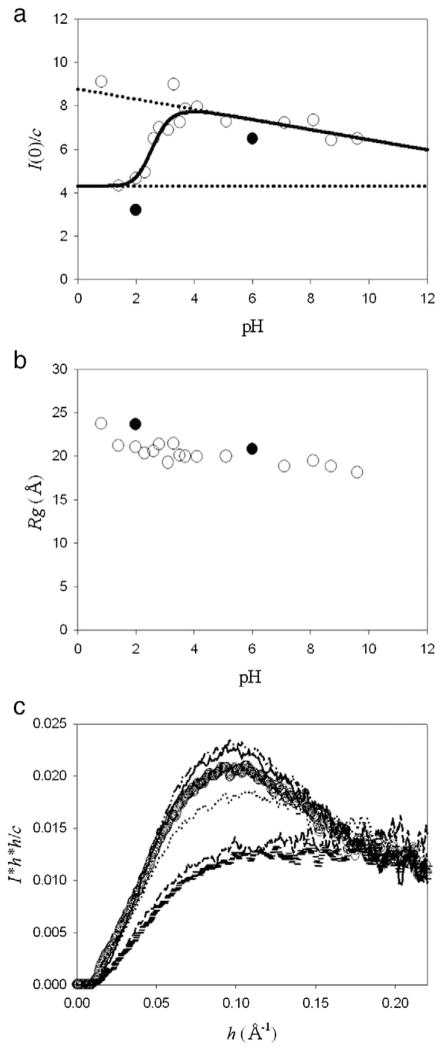
Fig. 3.
XSS experiments of hNck2 SH3 domain as a function of pH. Protein concentration was kept to be 2.9 to 3.9 mg/ml. At these concentrations, the protein forms dimer at pH 6, as described in the text (Fig. 1(c)). (a) I(0) / c plots against pH. A continuous line represents the theoretical curve based on Eq. (1) (except pH 0.8 and 3.3) [10]. Broken lines indicate the I(0) / c(M′) and I(0) / c(D′) values. The data were taken at Photon Factory (open circle) and SSRL (filled circle). About the data at SSRL (filled circle), the I(0) / c value at pH 2 was the average value of that at 1.2 and 1.4 mg/ml and that at pH 6 was also the average value from 1.0 to 2.8 mg/ml. At these concentrations also, the protein forms dimer at pH 6. (b) Rg dependence of pH. The data were taken at Photon Factory (open circle) and SSRL (filled circle). Rg at pH 2 and 6 at SSRL (filled circle) are the average value at 1.2 and 1.4 mg/ml and above 0.35 mg/ml, respectively. (c) Normalized Kratky plots of hNck2 SH3 domain at various pH. pH 1.4 (horizontal mark), pH 2.0 (dash), pH 2.6 (dot), pH 3.5 (circle), pH 4.1 (dash–dot–dot) and pH 7.1 (solid line), respectively.