Figure 3. Collagen V is tethered to the cell surface by HS GAG-independent interactions.
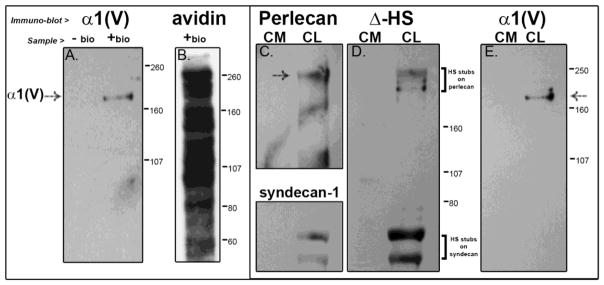
(A, E) The collagen V in the cultured cells is processed and in the mature form. (A) Further, presence of the α1 collagen V band in cell-surface biotinylated or “+ bio” (and absence in unbiotinylated or “− bio”) material from a streptavidin pull-down indicates that collagen V is a cell surface molecule of tendon fibroblasts. (B)This material is also shown blotted with streptavidin-HRP to show the full labeled complement of the numerous cell surface molecules. We further see that HS chains are not responsible for retaining collagen V at the cell surface; (C, D) even with efficient digestion of HS chains, as evidenced by reaction of the digested HSPGs perlecan (pericellular) and syndecan-1 (integral to the membrane) in panel C with the Δ-HS stub antibody in blot D, collagen V is still retained in the cell layer (panel E). Demarcations between panels indicate different exposures of a single gel to optimize signal for a specific protein. Separately bordered panels indicate different gels.
