Abstract
Importance
Grammatical comprehension difficulty is an essential supporting feature of the non-fluent/agrammatic variant of primary progressive aphasia (naPPA), but well-controlled clinical measures of grammatical comprehension are unavailable.
Objective
To develop a measure of grammatical comprehension and examine this comparatively in PPA variants and behavioural-variant frontotemporal degeneration (bvFTD) and to assess the neuroanatomic basis for these deficits with volumetric grey matter atrophy and whole-brain fractional anisotropy (FA) in white matter tracts.
Design
Case–control study.
Setting
Academic medical centre.
Participants
39 patients with variants of PPA (naPPA=12, lvPPA=15 and svPPA=12), 27 bvFTD patients without aphasia and 12 healthy controls.
Main outcome measure
Grammatical comprehension accuracy.
Results
Patients with naPPA had selective difficulty understanding cleft sentence structures, while all PPA variants and patients with bvFTD were impaired with sentences containing a centre-embedded subordinate clause. Patients with bvFTD were also impaired understanding sentences involving short-term memory. Linear regressions related grammatical comprehension difficulty in naPPA to left anterior-superior temporal atrophy and reduced FA in corpus callosum and inferior frontal-occipital fasciculus. Difficulty with centre-embedded sentences in other PPA variants was related to other brain regions.
Conclusions and relevance
These findings emphasise a distinct grammatical comprehension deficit in naPPA and associate this with interruption of a frontal-temporal neural network.
INTRODUCTION
Primary progressive aphasia (PPA) includes non-fluent/agrammatic (naPPA), semantic (svPPA) and logopenic (lvPPA) variants. Each has characteristic language deficits.1 Quantifying these abnormalities for disease-modifying treatment trials provides useful screening for histopathologic abnormalities.2 Effortful speech and limited grammatical expression, necessary features of naPPA, have been well documented in naPPA.3–6 Grammatical comprehension difficulty also is an important supporting feature of naPPA,1 but comparative studies of grammatical comprehension in small series are uncommon7–11 and anatomic assessments are rare.7,11
Grammatical comprehension involves long-distance syntactically specified relationships between words in a sentence. Patients with naPPA are impaired answering a simple question about a grammatically complex sentence,7 matching a complex sentence to one of several pictures,8 and arranging printed words into a question consistent with the content of a picture.12 While sensitive, these approaches have not proven specific and have not identified qualitatively distinct impairments in naPPA.7,8,11 Since grammatical comprehension of sentences may also involve working memory, it is important to dissociate these factors, particularly in patients who have working memory limitations.13,14 Non-aphasic patients with behavioural-variant frontotemporal degeneration (bvFTD) have executive deficits that interfere with narrative comprehension,15 but it is unclear whether this compromises grammatical comprehension.
Here, we examined grammatical comprehension difficulty in PPA variants and patients with bvFTD with a new clinical measure, and we related performance to regional grey matter (GM) and white matter (WM) abnormalities. Impaired grammatical comprehension has been related to left inferior frontal and anterior-superior temporal atrophy in two small series.7,11 We expected grammatically complex sentences with a cleft structure to be sensitive to grammatical difficulty in naPPA, and that this would be related to abnormalities in a GM–WM network involving inferior frontal and anterior-superior temporal regions.
METHODS
Participants
We studied 66 individuals recruited by experienced cognitive neurologists (DJI and MG) from the Department of Neurology at the University of Pennsylvania. This included 39 with variants of PPA (naPPA=12, lvPPA=15 and svPPA=12) and 27 non-aphasics with bvFTD. Two independent reviewers subclassified participants according to published criteria,1 based on medical history, neurologic examination and a detailed mental status evaluation. We excluded individuals with vascular disease, hydrocephalus, primary psychiatric disorder and medical illness that may impair cognition. Participants were age- and education-matched with 12 healthy, elderly controls. Clinical and demographic information is summarised in table 1. Subjects were further characterised by performance on neuropsychological measures of executive functioning, semantic memory and episodic memory. These measures are described in table 1, where performance is summarised.
Table 1.
Mean (±SD) demographic and clinical characteristics of healthy seniors and patients*
| Healthy controls n=12 | bvFTD n=27 | naPPA n=12 | lvPPA n=15 | svPPA n=12 | |
|---|---|---|---|---|---|
| Age (years) | 66.9 (6.9) | 62.0 (7.1) | 67.5 (10.1) | 66.2 (9.3) | 60.9 (7.9) |
| Education (years) | 16.2 (3.2) | 16.0 (3.0) | 14.4 (2.9) | 14.9 (3.5) | 16.3 (3.9) |
| MMSE (maximum=30) | 28.6 (1.2) | 25.6 (3.9) | 21.2 (9.7) | 20.2 (7.4) | 21.1 (5.8) |
| FAS task (words/3 min) | 45.5 (11.0) | 24.5 (13.2) | 15.3 (8.5) | 15.9 (10.6) | 15.2 (8.9) |
| Category naming task (words/3 min) | 42.8 (11.0) | 24.6 (10.6) | 31.1 (13.6) | 16.2 (10.1) | 13.2 (8.1) |
| Trail making test (maximum=26) | 25.0 (0.0) | 20.8 (8.3) | 18.9 (8.5) | 16.1 (7.7) | 21.3 (7.8) |
| Pyramid and palm tree Test (maximum=52) | 41.4 (13.7) | 44.8 (9.5) | 46.9 (6.0) | 44.8 (6.7) | 35.9 (6.8) |
| Boston naming test (maximum=30) | 28.8 (1.4) | 24.8 (4.3) | 24.6 (4.3) | 18.0 (9.5) | 7.3 (4.8) |
| Philadelphia verbal learning test | |||||
| Long delay (maximum=9) | 6.7 (2.0) | 4.2 (2.4) | 7.1 (2.6) | 3.5 (3.1) | 1.1 (1.6) |
| Digit span | |||||
| Forward (digits correct) | 7.3 (1.3) | 7.0 (1.5) | 6.3 (1.9) | 4.6 (1.8) | 6.2 (1.9) |
| Reverse (digits correct) | 5.3 (1.8) | 4.1 (1.9) | 3.2 (1.6) | 2.8 (1.1) | 4.0 (2.1) |
FAS task: A measure of fluency that requires subjects to list as many words that start with the letters F, A and S as they can, given 1 min for each category. Category naming test: A measure of category naming fluency that gives subjects 1 min to name as many items in each of three given categories (animals, tools and vegetables) as possible. Trail making test, part B: A measure of mental flexibility and executive function that requires subjects to connect randomly arranged circles labelled in numerical (1–13) and alphabetical (A–L) orders, alternating between numbers and letters. Pyramid and Palm Tree: A measure of semantic knowledge that requires subjects to choose which of two items is best associated with a target item; the task contains 52 words and 52 pictures, here averaged across the two modalities. Boston Naming Test: A measure of confrontation naming that requires the subject to name 30 illustrated stimuli. Philadelphia Verbal Learning Test: A measure of verbal episodic memory that monitors recall of nine words following five learning trials, an interference trial. Long delay assesses recall 30 min after initial presentation. Digit span: A measure of verbal short-term memory (forward digit span) and working memory (reverse digit span). BvFTD, behavioural-variant frontotemporal degeneration; lvPPA, logopenic variant primary progressive aphasia; MMSE; Mini Mental State Exam, naPPA, non-fluent/agrammatic primary progressive aphasia; svPPA, semantic variant primary progressive aphasia.
Behavioural materials
Sentence comprehension
We developed a two-alternative, forced-choice sentence-picture matching task. This is composed of 72 randomly ordered sentences and two colour pictures accompanying each sentence. The content of the sentences and the pictures included familiar, animate objects engaging in familiar activities. Forty-eight experimental sentences were equally divided into three factors with two levels of each factor, as illustrated in table 2. The structure factor included cleft (two propositions) and centre-embedded (three proposition) sentences; the length factor included short (nine words) or long (12 words, lengthened by a prepositional phrase strategically placed between grammatically linked sentence constituents) sentences; and the relativisation factor included subject-relative (agent-initial) and object-relative (patient-initial) sentences. Twenty-four control sentences did not contain a grammatical manipulation but were length-matched to experimental stimuli: half included a single main verb and the remainder were compound sentences concatenated by the conjunction ‘and’; half of each of these was short and half long. We found no statistical differences between these control versions and combined these for analyses reported below.
Table 2.
Examples of sentences used in the sentence comprehension task
| Type of sentence | Example |
|---|---|
| Control 1, short | The wild and tormented kid kicked the adventurous dog |
| Control 1, long | The interesting and funny woman in the room watched the humorous puppy |
| Control 2, short | The octopus was jolly and it trailed the seahorse |
| Control 2, long | The child with a smile was kind and she observed the kitten |
| Cleft, subject-relative, short | It was the caring dog that licked the cat |
| Cleft, subject-relative, long | It was the sneaky mouse with big ears that trailed the snake |
| Cleft, object-relative, short | It was the excited boy that the girl watched |
| Cleft, object-relative, long | It was the unpredictable man with good intentions that the woman poked |
| Centre-embedded, subject-relative, short | The fox that followed the domesticated cat was fierce |
| Centre-embedded, subject-relative, long | The boy with bad behaviour that pushed the tremendous lady was rude |
| Centre-embedded, object-relative, short | The woman that the smart man poked was athletic |
| Centre-embedded, object-relative, long | The kid in the kitchen that the calm mouse followed was fast |
Participants were shown a page containing two simple, coloured illustrations, one above the other. Both pictures illustrated the same two characters performing an action named in the associated sentence, with the characters switching thematic roles (agent and patient) in the two pictures (figure 1). The actors were semantically reversible and could perform the described action equally to each other. Adjectives in the sentences did not bias responses. Participants heard an oral sentence presented naturalistically and were asked to indicate the corresponding picture. We repeated a sentence once if requested by the participant. The top image was correct on half of each stimulus type. A practice session introduced participants to the materials and procedures, and all participants seemed to understand the task.
Figure 1.
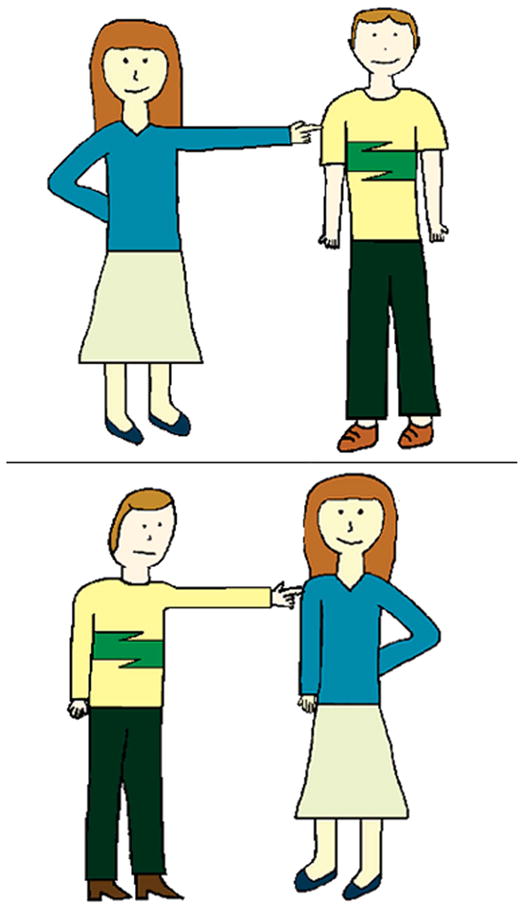
Sample task illustration. Note: 1. Example of a two-choice illustration used in the sentence task presented to subjects. Subjects indicated which of the two panels correctly illustrated an orally presented sentence. The sample shown here corresponds to the long, object-relative cleft sentence, ‘It was the unpredictable man with good intentions that the woman poked’.
Behavioural statistical analysis
Control participants performed near ceiling, so non-parametric statistics were used, including Friedman tests to determine interactions of group with structure, length and relativisation factors and Wilcoxon tests to analyse each group’s comprehension performance more closely. All analyses were conducted using a two-tailed α of 0.05.
Imaging methods
We related sentence comprehension performance directly to T1 and diffusion tensor imaging (DTI) MRI scans available in 46 participants (naPPA, n=6; lvPPA, n=10; svPPA, n=8; and bvFTD, n=22).
GM atrophy
Details of imaging methods for GM density, reported previously,16 are provided in online supplementary appendix 1. Volumetric T1 images were collected at 3 T with 1 mm isotropic voxels. We performed voxel-wise comparisons between patients and controls with two-sample t-tests using a false discovery rate (FDR)-corrected height threshold. Since GM disease varies depending on underlying pathology17 and the number of participants in a group, we minimised these potential sources of bias by selecting statistical thresholds that equated the volume of atrophy across groups (naPPA: q<0.025; lvPPA: q<0.001; svPPA: q<0.0005; bvFTD: q<0.00001). We identified clusters surviving an extent threshold of 50 adjacent voxels. Linear regression related GM density to impaired language performance at a height threshold of p<0.05 (uncorrected), an extent threshold of 10 voxels and a peak voxel level in each cluster of p<0.001 unless otherwise noted.
WM fractional anisotropy
Details of the imaging methods for WM fractional anisotropy (FA), reported previously,16 are provided in online supplementary appendix 1. Briefly, diffusion-weighted images were acquired at 3 T with either a 30-direction or 12-direction acquisition sequence. To minimise potential bias associated with a DTI sequence, we included a nuisance covariate for DTI sequence in all analyses. To identify areas of reduced FA, we performed two-sample t-tests comparing patients and controls at a height threshold of q<0.01 (FDR-corrected) and an extent threshold of 200 adjacent voxels. Linear regression-related reduced FA to impaired language performance at a height threshold of p<0.005 (uncorrected), an extent threshold of 50 voxels and a peak voxel level of p<0.001.
RESULTS
Behavioural results
A Friedman test analysing all groups’ scores for structure, length and relativisation factors demonstrated an interaction effect (χ2(6)=372.2; p<0.0001). Figure 2 illustrates comprehension accuracy for each factor. Interaction effects for each group and factor are presented below.
Figure 2.
Mean (+SE) percent correct sentence comprehension performance. Notes: (1) (A) Percentage of correct control, cleft, and centre-embedded sentences by diagnosis. (B) Percentage of correct short and long sentences by diagnosis. (C) Percentage of correct subject- and object-relative sentences by diagnosis. For these graphs, error bars represent the SE of the mean. (2) * denotes a significant difference in mean scores (see text for details). In (A), cleft and centre-embedded differences are not shown. (3) NCs, normal controls; naPPA, non-fluent/agrammatic primary progressive aphasia (PPA); lvPPA, logopenic variant PPA; svPPA, semantic variant PPA; bvFTD, behavioural variant frontotemporal degeneration.
Structure
A Friedman test demonstrated a group–structure interaction (χ2(2)=43.1; p<0.001). Follow-up Wilcoxon tests in naPPA demonstrated worse performance with cleft than control sentences (Z=−1.992; p=0.046). Only naPPA showed this difference, and this was not evident in other groups including lvPPA (Z=−1.063, p=0.28). The deficit with cleft sentences in naPPA was equally evident for short and long versions (p>0.10) and subject- and object-relative versions (p>0.10). This could not be attributed easily to non-specific cognitive difficulty since sentence comprehension did not correlate with MMSE in naPPA, although MMSE was correlated with comprehension in other patient groups (all Spearman correlations p<0.025). All patient groups were worse with centre-embedded than control sentences (all contrasts p<0.05), and all groups except svPPA demonstrated lower scores on centre-embedded than cleft sentences (≥=−2.533; p=0.011). All groups showed worse performance for object-relative than subject-relative versions of centre-embedded sentences (all contrasts p<0.01), and all groups were equally impaired for long and short versions of centre-embedded sentences.
Length
While a Friedman test did not show an interaction between group and length (χ2(1)=1.67; p=0.197), we examined this factor more closely because of hypotheses that short-term memory may affect performance. A Wilcoxon test demonstrated difficulty with long compared to short sentences only in bvFTD (Z=−2.226; p=0.026). In bvFTD, sentence comprehension correlated with measures of executive functioning, including FAS fluency, category naming fluency and reverse digit span (all Spearman correlations p<0.001). A length effect was not evident in other groups.
Relativisation
Although a Friedman test demonstrated a group×relativisation interaction effect (χ2(1)=45.6; p<0.001), this was due to controls’ near-ceiling performance on object-relative sentences. Mann–Whitney tests showed worse performance on object relatives in all patient groups compared with controls (all contrasts p<0.005), and all groups had worse performance for object relatives than subject-relatives (all contrasts p<0.05).
Imaging results
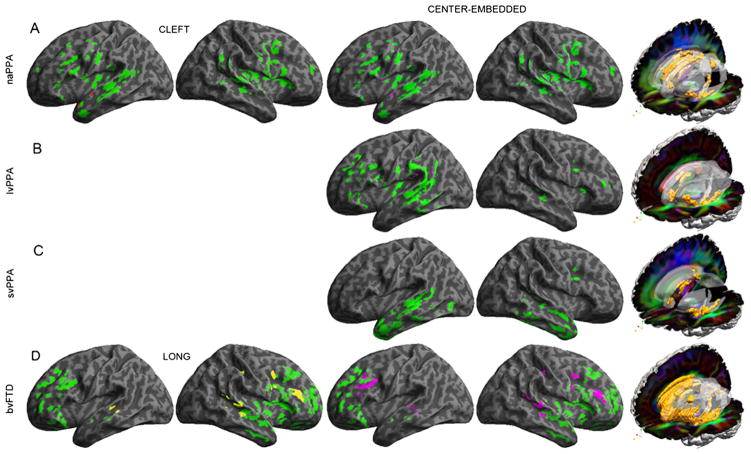
Significantly reduced GM density and regressions relating GM density to comprehension are illustrated in figure 3. Figure 3 also shows significantly reduced FA in WM tracts. Peak coordinates for GM atrophy and reduced FA in WM are summarised in online supplementary appendix 2. Regression analyses relating comprehension performance to GM atrophy and reduced FA in WM are summarised in table 3.
Figure 3.
Grey matter atrophy and regressions relating behaviour to atrophy, and reduced white matter fractional anisotropy. Note: (1) Images on the left illustrate whole brain renderings of grey matter atrophy, and coloured areas show the specific anatomic distribution of significant atrophy compared to matched controls. Anatomic locations of the corresponding clusters are provided in online supplementary appendix 2. Areas in colours other than green illustrate loci of significant regressions relating performance to grey matter atrophy. Images on the right illustrate reduced fractional anisotropy on red-green-blue (RGB) diffusion tensor images (diffusion orientation: red=left-right, green=anterior-posterior, blue=superior-inferior). Ghost areas indicate anatomic regions and orange areas within these ghosts indicate areas of significantly reduced fractional anisotropy compared to matched controls. Anatomic locations of the corresponding clusters are provided in online supplementary appendix 2. (A): non-fluent/agrammatic primary progressive aphasia; (B): logopenic variant primary progressive aphasia; (C): semantic variant primary progressive aphasia; (D): behavioural variant frontotemporal degeneration.
Table 3.
Regressions of language performance with grey matter atrophy and reduced white matter fractional anisotropy
| MNI coordinates
|
|||||
|---|---|---|---|---|---|
| Anatomic locus (Brodmann area) | x | y | z | Z score | Cluster size (voxels) |
| Grey matter atrophy regressions | |||||
| naPPA cleft | |||||
| L anterior-superior temporal (21) | −64 | −6 | −16 | 3.37 | 98 |
| naPPA centre-embedded | |||||
| L anterior-superior temporal (38) | −56 | 14 | −18 | 2.96* | 182 |
| lvPPA centre-embedded | |||||
| L posterior-superior temporal (42) | −50 | −28 | 16 | 3.18 | 57 |
| L inferior parietal (39) | −44 | −54 | 28 | 3.11 | 12 |
| svPPA centre-embedded | |||||
| L middle temporal (21) | −66 | −38 | −16 | 3.00* | 37 |
| bvFTD long | |||||
| L prefrontal (9) | −34 | 22 | 28 | 3.09* | 204 |
| L anterior cingulate (24) | −4 | 4 | 38 | 2.98* | 110 |
| L superior temporal (22) | −54 | −34 | 4 | 3.72 | 58 |
| R prefrontal (46) | 44 | 26 | 22 | 3.75 | 229 |
| R inferior frontal (6) | 36 | 4 | 34 | 3.21 | 170 |
| R superior temporal (22) | 54 | −42 | 10 | 3.62 | 48 |
| R middle temporal (21) | 50 | −38 | 4 | 3.24 | 124 |
| R postcentral (2) | 44 | −26 | 38 | 4.32 | 42 |
| bvFTD centre-embedded | |||||
| L prefrontal (9) | −34 | 22 | 28 | 3.44 | 232 |
| L prefrontal (46) | −28 | 46 | 18 | 3.07* | 37 |
| L superior temporal (22) | −54 | −36 | 2 | 3.47 | 58 |
| R inferior frontal (6) | 50 | 2 | 28 | 3.64 | 213 |
| R prefrontal (46) | 44 | 26 | 22 | 3.64 | 242 |
| R superior temporal (22) | 54 | −42 | 10 | 3.26 | 47 |
| R middle temporal (21) | 50 | −40 | 4 | 3.48 | 144 |
| R postcentral (2) | 44 | −26 | 38 | 4.51 | 39 |
| Reduced white matter fractional anisotropy regressions | |||||
| naPPA cleft | |||||
| L corpus callosum (body-anterior)/corona radiata | −17 | 8 | 30 | 4.14 | 187 |
| naPPA centre-embedded | |||||
| L inferior frontal-occipital | −33 | −43 | 16 | 3.75 | 90 |
| lvPPA centre-embedded | |||||
| L inferior frontal-occipital | −24 | 19 | 0 | 4.36 | 66 |
| L inferior longitudinal | −36 | −56 | −2 | 3.54 | 195 |
| R corpus callosum (genu) | 14 | 29 | 2 | 3.69 | 122 |
| R corpus callosum (body-middle) | 14 | −11 | 36 | 3.21 | 74 |
| svPPA centre-embedded | |||||
| L inferior longitudinal | −36 | −59 | 4 | 4.00 | 127 |
| emsp;bvFTD long | |||||
| L corpus callosum (body-middle)/corona radiata | −18 | −8 | 42 | 3.28 | 68 |
| R corpus callosum (body-posterior)/cingulum | 14 | −20 | 30 | 3.59 | 128 |
| B corpus callosum (body-middle) | 0 | 1 | 28 | 3.43 | 154 |
| bvFTD centre-embedded | |||||
| L inferior frontal-occipital | −35 | −12 | −6 | 3.24 | 65 |
| L corpus callosum (body-middle)/corona radiata | −18 | −8 | 42 | 3.12 | 57 |
| R corpus callosum (body-posterior)/corona radiata | 14 | −20 | 30 | 3.17 | 68 |
| R corpus callosum (genu)/corona radiata | 20 | 32 | 6 | 3.11 | 67 |
indicates a cluster peak for a regression relating performance to atrophy at a level approaching significance (p<0.002) in a hypothesised area. bvFTD, behavioural-variant frontotemporal degeneration; lvPPA, logopenic variant primary progressive aphasia; naPPA, MNI; Montreal Neurological Institute, non-fluent/agrammatic primary progressive aphasia; svPPA, semantic variant primary progressive aphasia
GM atrophy in naPPA was centred in left inferior frontal, insula and anterior-superior temporal regions, extending to right frontal regions. Regression analyses related impaired comprehension for cleft and centre-embedded sentences to GM atrophy in left anterior-superior temporal cortex. Reduced FA was evident in left superior longitudinal fasciculus (SLF) and inferior frontal-occipital fasciculus (IFO) as well as corpus callosum (CC), fornix and uncinate fasciculus (UNC). Regressions related performance with cleft sentences to reduced FA in left anterior CC and related performance with centre-embedded sentences to left IFO.
In lvPPA, GM atrophy was centred in left posterior-superior temporal and inferior parietal regions, extending to left frontal areas and minimally to the right hemisphere. Regression analyses related impaired centre-embedded comprehension to GM atrophy in left posterior-superior temporal and inferior parietal regions. Reduced FA was seen in left IFO, left inferior longitudinal fasciculus (ILF), fornix and CC. Regressions related impaired centre-embedded sentence comprehension to reduced FA in left IFO, left ILF and CC.
In svPPA, GM atrophy was most prominent in left anterior temporal regions, extending posteriorly into left temporal areas and involving the right anterior temporal lobe. Regression analyses related impaired centre-embedded comprehension to GM atrophy in left mid-lateral temporal regions. Reduced FA in svPPA was seen in left ILF, UNC and CC. Impaired centre-embedded comprehension was related to reduced FA in left ILF.
In bvFTD, GM atrophy was evident throughout frontal and anterior temporal lobes bilaterally. Regression analyses related impaired centre-embedded comprehension to frontal and superior temporal GM atrophy bilaterally. Impaired long sentence comprehension also was related to bilateral frontal and superior temporal GM atrophy. Reduced FA was seen in multiple WM regions bilaterally. Impaired centre-embedded comprehension was related to reduced FA in CC, bilateral SLF and left IFO. Difficulty with long sentences was related to reduced FA in bilateral CC.
DISCUSSION
Grammatical comprehension plays a crucial role in the diagnosis of naPPA.18 This study developed a new method to assess grammatical comprehension deficits in naPPA and help characterise this difficulty in other PPA variants as well as bvFTD. We found a deficit understanding cleft sentences only in naPPA, and this was related to interruption of a frontal-temporal neural network for sentence processing. All FTD subgroups were significantly impaired with centre-embedded sentences involving greater resource demands, and patients with bvFTD were compromised with the short-term memory component of sentences. Linear regression analyses related impairments with these sentence materials to other anatomic regions.
Development of the grammatical task
naPPA, also known as progressive non-fluent aphasia, has been associated with a grammatical comprehension deficit since its earliest description.19 This observation has led to its inclusion as an important supplementary feature of naPPA in a recent consensus report.1 Unfortunately, several confounds have limited the informativeness of this feature in prior assessments. One problem has been the use of highly demanding grammatical materials to demonstrate a deficit in grammatical comprehension. Using centre-embedded subordinate phrase constructions, for example, comparative studies found a broad sentence comprehension deficit across all PPA variants.7,8 The present study confirmed this impairment in a broad spectrum of FTD. More importantly, cleft sentences were selectively impaired in naPPA and were not significantly impaired in other patient groups. These sentences were less difficult statistically than centre-embedded sentences. This may reflect that cleft sentences have fewer resource demands because they contain only two propositions, while centre-embedded sentences contain three. While non-specific cognitive difficulty may have contributed to comprehension impairments in lvPPA, svPPA and bvFTD, we did not find a correlation between MMSE and comprehension performance in naPPA.
A second problem that may have confounded prior attempts to identify a measure specific for naPPA is related to task demands. Challenging tasks used to ascertain sentence comprehension difficulty have included forming a wh- question using an anagram task,12 or holding a target sentence in mind while answering a question about the target sentence.7 To minimise task-related demands, several studies have demonstrated selective comprehension impairments in naPPA with online measures.10,20 However, it is difficult to develop an online task that can be administered in a clinical setting. Using a balanced approach that minimises task-related demands and considers the practical constraints of task administration by asking patients to relate a sentence to one of two pictures (also see ref. 6), we identified a selective deficit for cleft sentences in naPPA. The findings presented in this paper demonstrate the efficacy of the task, even in patients with mild disease, and we are continuing to administer this test to a larger PPA cohort in order to confirm the findings of this study.
Grammatical comprehension deficits in naPPA
Patients with naPPA have disease centred in left inferior frontal and adjacent anterior-superior temporal regions.11,16,21,22 Grammatical expression deficits have been related to this anatomic distribution of disease.5 However, studies directly relating sentence comprehension to regional GM atrophy in naPPA have been very rare. One study associated impaired sentence comprehension with posterior-inferior frontal and anterior-superior temporal regions of the left hemisphere.7 In mixed groups of neurodegenerative patients, grammatical comprehension was related to left inferior frontal atrophy.23,24 The present study found that difficulty understanding cleft sentences in naPPA is related to left anterior-superior temporal GM atrophy. Although we had a small number of imaging datasets, this was essentially the same anatomic distribution related to impairments with centre-embedded sentences, confirming the reliability of cleft sentences for detecting grammatical comprehension deficits in naPPA. Future work will have to confirm this anatomic locus with larger numbers of patients. Previous work has suggested that this anterior-superior temporal region is part of a ventral WM projection stream25 important for processing grammatical information in naPPA.16 Consistent with this view, we observed reduced FA in left IFO in naPPA, as described previously,26 and difficulty with centre-embedded sentences also was related to reduced FA in this tract. We observed reduced FA in left SLF in naPPA as well, as observed previously.16,26,27 Although regression analyses did not implicate this tract directly in naPPA sentence processing difficulty, reduced FA in the nearby anterior CC and corona radiata was related to comprehension of cleft sentences. Much evidence in the stroke literature implicates callosal dysfunction in performance on grammatical measures in Broca’s aphasia,28–30 and other studies have related grammatical comprehension difficulty in naPPA directly to SLF.24 The discrepancy between findings of WM involvement in Broca’s aphasia and naPPA may have been due to different groups of patients and different sentence materials.
Sentence comprehension deficits in bvFTD
Patients with bvFTD were significantly impaired with centre-embedded sentences. We also used a fully penetrated design to assess an effect for length. Only patients with bvFTD showed a length effect as well. Despite the absence of an obvious aphasia, language-related deficits have been reported on comprehension measures in bvFTD involving narrative15 and discourse.31 Resource-related limitations were implicated in these deficits, and the present study found a correlation between impaired sentence comprehension and executive resource limitations in bvFTD as well. Additional work is needed to assess more precisely the role of short-term memory deficits during sentence processing in bvFTD.
bvFTD patients’ difficulty with centre-embedded sentences was related to extensive atrophy in several frontal and temporal regions. Since most of the statistically significant regressions were in the right hemisphere, we suspect that this comprehension deficit in bvFTD was not primarily linguistic in nature, but was related instead to the resource limitations that contributed to their comprehension deficits. Consistent with this view, many of the same GM regions implicated in subjects’ grammatical comprehension deficit were associated with their difficulty understanding lengthy sentences. Among these were prefrontal areas important for strategic organisation, the anterior cingulate region important for attention and initiation and posterior-superior temporal and inferior parietal regions important for short-term memory. Consistent with the bilateral nature of the brain regions implicated in the patients’ grammatical comprehension difficulty, extensive correlations related comprehension performance to WM disease in CC and corona radiata. Moreover, similar WM regions were implicated in grammatical and resource-related aspects of sentence comprehension. This suggests a common source of impairment across both aspects of sentences. Additional work correlating measures of executive functioning with WM disease is needed to confirm these observations.
Sentence comprehension deficits in lvPPA and svPPA
We found a deficit for centre-embedded sentences in lvPPA. While a preliminary report described a deficit for lengthy sentences in lvPPA,8 we did not find this. This discrepancy may have been due in part to differences in the materials used in these studies. We found left temporal-parietal atrophy in lvPPA, as described elsewhere.14,32 Atrophy in this GM distribution was related directly to difficulty understanding centre-embedded sentences. We also found that reduced FA was related to grammatical comprehension difficulty in IFO and CC, as we found in naPPA. These observations suggest an alternate explanation for sentence comprehension difficulty in lvPPA that is independent of a short-term memory deficit, and instead involves interruption of a large-scale, frontal-temporal sentence processing network. This will require evaluation by additional studies.
Poor comprehension of centre-embedded sentences was found in svPPA as well. While previous work associated sentence comprehension difficulty with a fundamental deficit in lexical comprehension,7,33 we did not find that sentence comprehension correlates with the Pyramid and Palm Tree assessment of lexical and object comprehension. Nevertheless, impaired centre-embedded comprehension was related to GM atrophy in left mid-lateral temporal cortex and to reduced FA in the associated left ILF. This network has been implicated in lexical processing in svPPA.34 Although beyond the scope of the present study, assessments of other aspects of lexical processing are needed before dismissing the association of grammatical comprehension with lexical processing.
Supplementary Material
Acknowledgments
Funding This work was supported in part by National Institutes of Health (AG017586, NS044266, AG032953, AG038490, NS053488, AG015116).
Footnotes
Competing interests None.
Ethics approval University of Pennsylvania Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Contributors DC: Drafting, language analysis, statistical analysis. CO: Acquisition, statistical analysis. JP: Acquisition, imaging statistical analysis. SA: Language analysis. DI: Acquisition. CM: Imaging analysis. KR: Statistical analysis. MG: Drafting, study concept, imaging analysis, statistical analysis, funding.
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman M. Primary progressive aphasia: clinical-pathological correlations. Nat Rev Neurol. 2010;6:88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ash S, Moore P, Vesely L, et al. Non-fluent speech in frontotemporal lobar degeneration. J Neurolinguistics. 2009;22:370–83. doi: 10.1016/j.jneuroling.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogalski E, Cobia D, Harrison TM, et al. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76:1804–10. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunawardena D, Ash S, McMillan C, et al. Why are patients with progressive nonfluent aphasia nonfluent? Neurology. 2010;75:588–94. doi: 10.1212/WNL.0b013e3181ed9c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson SM, Dronkers NF, Ogar JM, et al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J Neurosci. 2010;30:16845–54. doi: 10.1523/JNEUROSCI.2547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peelle JE, Troiani V, Gee JC, et al. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics. 2008;21:418–32. doi: 10.1016/j.jneuroling.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson SM, Galantucci S, Tartaglia MC, et al. The neural basis of syntactic deficits in primary progressive aphasia. Brain Lang. 2012;122:190–8. doi: 10.1016/j.bandl.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc. 1996;2:511–24. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- 10.Grossman M, Rhee J, Antiquena P. Sentence processing in frontotemporal dementia. Cortex. 2005;41:764–77. doi: 10.1016/s0010-9452(08)70295-8. [DOI] [PubMed] [Google Scholar]
- 11.Mesulam MM, Wieneke C, Rogalski E, et al. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66:1545–51. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weintraub S, Mesulam MM, Wieneke C, et al. The Northwestern Anagram Test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009;24:408–16. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libon DJ, Xie SX, Moore P, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–75. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–34. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farag C, Troiani V, Bonner M, et al. Hierarchical organization of scripts: converging evidence from fMRI and frontotemporal degeneration. Cereb Cortex. 2010;20:2453–63. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman M, Powers JM, Ash S, et al. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang. 2013 doi: 10.1016/j.bandl.2012.10.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMillan CT, Irwin DJ, Avants BB, et al. White matter imaging helps dissociate tau from TDP-43 in frontotemporal degeneration. J Neurol Neurosurg Psychiatry. 2013 doi: 10.1136/jnnp-2012-304418. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11:545–55. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman M, Mickanin J, Onishi K, et al. Progressive non-fluent aphasia: language, cognitive and PET measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci. 1996;8:135–54. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- 20.Peelle JE, Cooke A, Moore P, et al. Syntactic and thematic components of sentence processing in progressive nonfluent aphasia and nonaphasic frontotemporal dementia. J Neurolinguistics. 2007;20:482–94. doi: 10.1016/j.jneuroling.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogalski E, Cobia D, Harrison TM, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–50. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestor PJ, Graham NL, Fryer TD, et al. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–18. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- 23.Amici S, Brambati SM, Wilkins DP, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007;27:6282–90. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson SM, Galantucci S, Tartaglia MC, et al. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72:397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev. 2011;91:1357–92. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- 26.Schwindt GC, Graham NL, Rochon E, et al. Whole-brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum Brain Mapp. 2013;34:973–84. doi: 10.1002/hbm.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galantucci S, Tartaglia MC, Wilson SM, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134:3011–29. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winhuisen L, Thiel A, Schumacher B, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia. Stroke. 2005;36:1759–63. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- 29.van Oers CAMM, Vink M, van Zandvoort MJE, et al. Contribution of the left and right inferior frontal gyrus in recovery from aphasia. A functional MRI study in stroke patients with preserved hemodynamic responsiveness. Neuroimage. 2010;49:885–93. doi: 10.1016/j.neuroimage.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton RH, Sanders LD, Benson J, et al. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. 2010;113:45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan CT, Rascovsky K, Khella MC, et al. The neural basis for establishing a focal point in pure coordination games. Soc Cogn Affect Neurosci. 2012;7:881–7. doi: 10.1093/scan/nsr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohrer JD, Ridgway GR, Crutch SJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2009;49:984–93. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochon E, Kave G, Cupit J, et al. Sentence comprehension in semantic dementia: a longitudinal case study. Cogn Neuropsychol. 2004;21:317–30. doi: 10.1080/02643290342000357. [DOI] [PubMed] [Google Scholar]
- 34.Agosta F, Henry RG, Migliaccio R, et al. Language networks in semantic dementia. Brain. 2010;133:286–99. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.