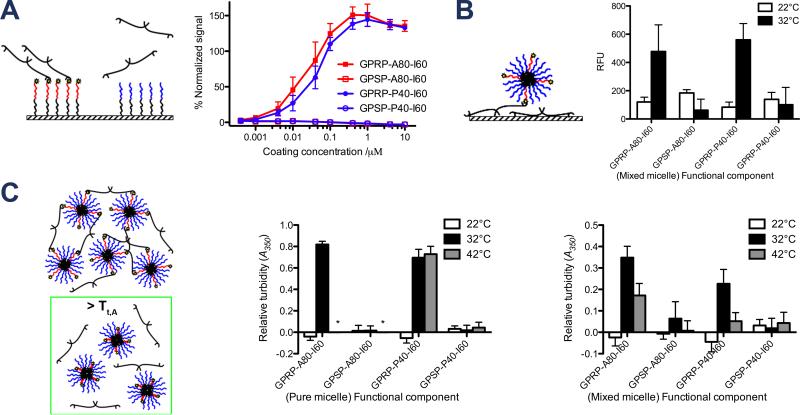
Figure 6.
Characterization of ELP affinity for fibrinogen as a function of temperature. (A) Specific binding of fibrinogen to immobilized GPRP-A80-I60 via the fibrinogen-binding peptide in an ELISA setup. ELISA plate readouts (λ = 450 nm) were normalized to GPRPFPAC-coated control wells and reported as % normalized signal as a function of coating concentration. (B) Temperature-responsive binding of fluorescently-labeled mixed micelles to immobilized fibrinogen in 96-well plate setup. Fluorescence readouts reflecting the amount of GPSP-P40-I60-AF546 recruited to the immobilized fibrinogen by the indicated unlabeled “functional component” at 22°C and 32°C are reported as relative fluorescent units (λEx = 540 nm, λEm = 573 nm). (C) Temperature-responsive binding between micelles and fibrinogen in solution at 22°C, 32°C, 42°C. Absorbance readouts reflecting the interaction of the pure micelles (comprising the indicated ELP diblocks only) or mixed micelles (1:4 mixtures of the indicated functional component with GPSP-P40-I60) with fibrinogen are reported as relative turbidity (λ = 350 nm). Asterisks indicate wells in which the ELP-only controls precipitated out.