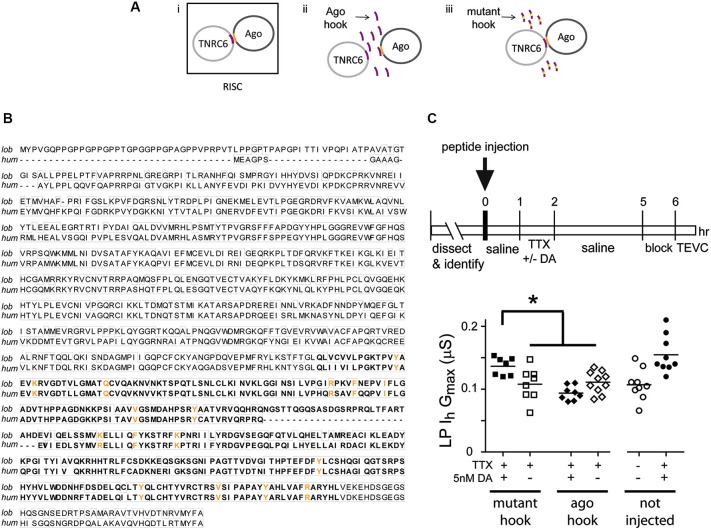
Figure 5.
A functional RNAi pathway is necessary for the DA- and activity-dependent increase in LP IhGmax. (A) i. The Ago hook peptide on the TNRC6 protein (purple) binds amino acids in the PIWI domain of the Ago protein (orange); the dimer is a component of the multiprotein complex, RISC, which is an essential element in the RNAi pathway. Additional RISC proteins are not shown. ii. Ago hook peptide competes with TNRC6 for binding to Ago, and excess Ago hook peptide disrupts RISC formation and the RNAi pathway. iii. Mutating amino acids in the Ago hook prevents it from binding to Ago, and the TNRC6-Ago dimer forms in the presence of excess mutant hook. (B) Alignment of lobster (KF602070) and human (AF093097) Ago1 proteins. Identical amino acids are boxed. The PIWI domain involved in binding TNRC6 is bolded. Amino acids necessary for binding to TNRC6 are shown in orange. (C) Injecting hook, but not mutant hook peptide into the LP neuron prevented the DA-induced, activity-dependent persistent increase in LP Ih Gmax. The upper panel shows the experimental protocol. The lower panel plots LP Ih Gmax for each treatment. Each symbol is one experiment; the horizontal bars are the means. Asterisk indicates significant differences using a one-way ANOVA with Tukey’s post hoc tests that made all pairwise comparisons, F(3,29) = 7.036, p = 0.0011. Uninjected control and DA-treated preparations from experiments in Figure 3 are shown for comparison.