Fig. 3.
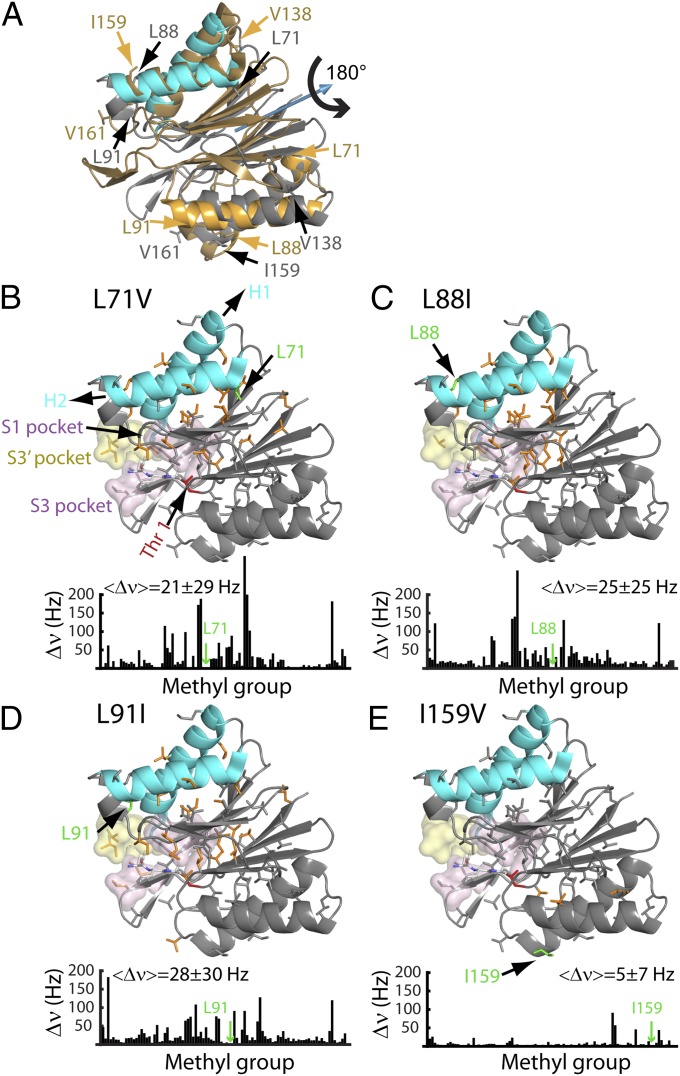
An allosteric pathway regulating the function of HslV. (A) The structure of each HslV monomer is approximately symmetric about a pseudotwofold axis that runs through the center of the protein, which is indicated by the blue arrow. Shown in gray (cyan for H1/H2) is the structure of an HslV monomer using the same orientation as in Fig. 1, right subunit, whereas the structure obtained by applying a 180° rotation about the symmetry axis (blue arrow) is indicated in gold. The positions of some of the mutations used are indicated. (B–E) Chemical shift changes relative to the WT protein are indicated in bar chart form and color-coded on the structure. Each methyl-containing residue is depicted in stick representation, and those methyls that undergo changes in shift ≥25 Hz are highlighted in orange. The mean chemical shift change (±1 SD) is indicated, excluding methyl groups ≤ 8 Å from the site of mutation. Note that the L88I mutation (H2) shows large changes in chemical shifts, whereas the mutation involving the symmetry-related position I159V shows little change. Where changes are observed, they extend from helices H1/H2 to regions surrounding the active site and/or substrate binding sites (S1, S3, and S3′) as indicated.