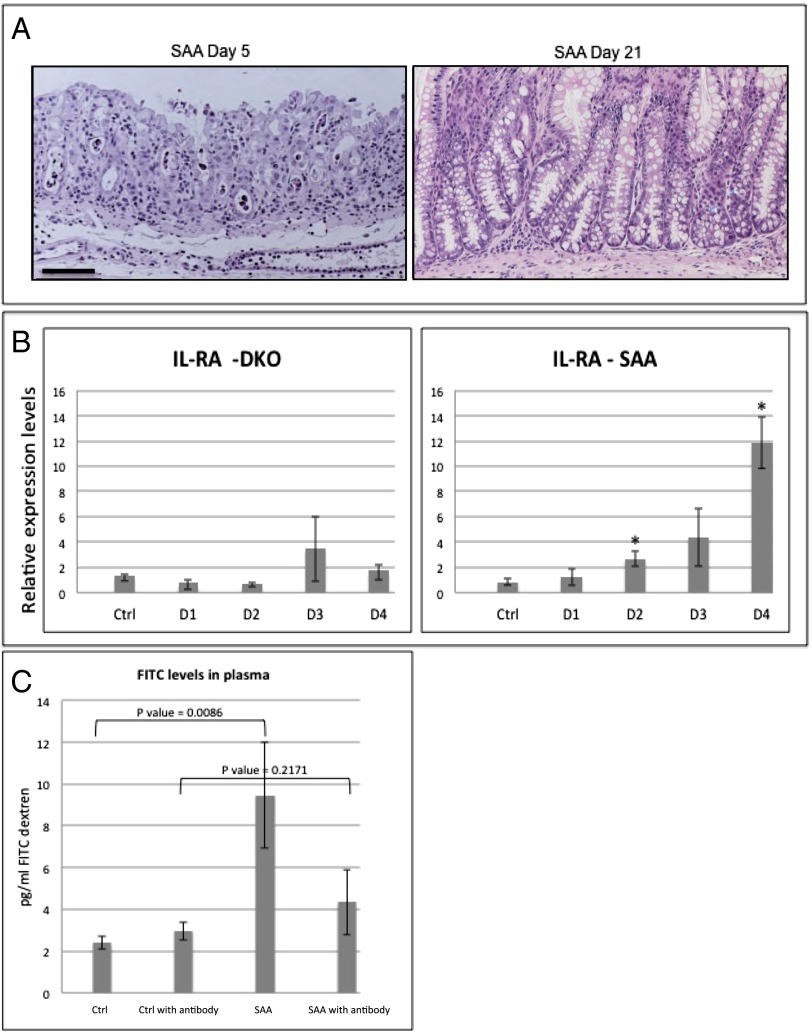
Fig. 6.
Mucositis mouse model implies IL-1β as an optional therapeutic target for the treatment of mucositis. (A) H&E staining of SAA colons at days 5 and 21. SAA mice recover from the pathologic condition induced by β-TrCP2 KO induction and present nearly normal gut histology 3 wk after KO induction. (B) qPCR analysis of the endogenous IL-1β antagonist IL-Ra, a well-characterized NF-κB target gene, 1, 2, 3, and 4 d after β-TrCP KO induction. No up-regulation is observed in the DKO mice (n = 2 for each day, P = 0.2374 vs. control at day 1, P = 0.1068 vs. control at day 2, P = 0.2586 vs. control at day 2, and P = 0.6802 vs. control at day 4), which have no NF-κB activation as a result of stabilized IκB. However, in SAA mice (n = 3 for each day; P = 0.4165 vs. control at day 1, P = 0.0089 vs. control at day 2, P = 0.0551 vs. control at day 3, and P = 0.0008 vs. control at day 4), with intact NF-κB, IL-RA is up-regulated following β-TrCP KO induction. (C) FITC levels in the plasma of the indicated mice 6 h after FITC-dextran force-feeding. SAA mice show fourfold increase in FITC levels, indicating high gut permeability. However, SAA treated with anti–IL-1β neutralizing antibody (daily i.p. injections, 400 mg/kg body weight) showed reduced levels of plasma FITC, comparable with the control mice, revealing the critical role of IL-1β in the barrier disruption process of these mice even in the presence of intact NF-κB signaling (P values are indicated).