Significance
Most vaccines confer immunity by eliciting long-term production of antibodies that bind to and neutralize the vaccine antigen. Remarkably, very little is known regarding the identities, sequence diversity, relative concentrations, or binding functionalities of the mAbs that comprise the serum repertoire elicited by vaccination. Here, we have delineated the constituent antibodies of the human serum IgG repertoire after vaccination and examined their relationship to the antibody V gene repertoire encoded by circulating B cells. The results detail the molecular composition and characteristics of the vaccine-specific serum antibody repertoire and demonstrate differences between the end-point response (the serum antibodies) and the peripheral B cells responding to the vaccine.
Keywords: B-cell repertoire, proteomics
Abstract
Most vaccines confer protection via the elicitation of serum antibodies, yet more than 100 y after the discovery of antibodies, the molecular composition of the human serum antibody repertoire to an antigen remains unknown. Using high-resolution liquid chromatography tandem MS proteomic analyses of serum antibodies coupled with next-generation sequencing of the V gene repertoire in peripheral B cells, we have delineated the human serum IgG and B-cell receptor repertoires following tetanus toxoid (TT) booster vaccination. We show that the TT+ serum IgG repertoire comprises ∼100 antibody clonotypes, with three clonotypes accounting for >40% of the response. All 13 recombinant IgGs examined bound to vaccine antigen with Kd ∼ 10−8–10−10 M. Five of 13 IgGs recognized the same linear epitope on TT, occluding the binding site used by the toxin for cell entry, suggesting a possible explanation for the mechanism of protection conferred by the vaccine. Importantly, only a small fraction (<5%) of peripheral blood plasmablast clonotypes (CD3−CD14−CD19+CD27++CD38++CD20−TT+) at the peak of the response (day 7), and an even smaller fraction of memory B cells, were found to encode antibodies that could be detected in the serological memory response 9 mo postvaccination. This suggests that only a small fraction of responding peripheral B cells give rise to the bone marrow long-lived plasma cells responsible for the production of biologically relevant amounts of vaccine-specific antibodies (near or above the Kd). Collectively, our results reveal the nature and dynamics of the serological response to vaccination with direct implications for vaccine design and evaluation.
Most approved vaccines confer protection against infectious diseases by the induction of long-lived plasma cells (LLPCs), which secrete antibodies that serve to neutralize and opsonize the pathogen for many years or decades (1–3). Additionally, the generation of memory B cells (mBCs) provides both a mechanism for the rapid synthesis of affinity matured, antigen-specific antibodies following rechallenge and a means to diversify the humoral immune response to confer protection against rapidly evolving viruses or bacteria (4). Although some vaccines elicit antibody titers that remain virtually constant for many decades, for others, including the tetanus toxoid (TT) vaccine, antibody titers wane monotonically over time (5). Booster immunization triggers the rapid expansion and differentiation of cognate B cells, generating antigen-specific plasmablasts that peak in concentration in peripheral blood after 6–7 d and subsequently rapidly decline to nearly undetectable levels (6, 7). Some, but not all, of these peak-wave plasmablasts migrate to specialized niches overwhelmingly located in the bone marrow (BM) and survive as LLPCs (8), which constitute the major source of all classes of Ig in the serum (9).
The establishment of serological memory following either primary or booster vaccination is not understood well (10–14). Even though antibody production is the most critical effector function of B-cell immunity, and antigen-specific antibodies in the serum play a key role in protection against pathogen challenge, technical difficulties have precluded direct determination of the identities of the mAbs that comprise the serum antibody response to vaccination. However, recent studies showing that flu vaccination elicits not only neutralizing antibodies but also antibodies that enhance infection by different flu strains (15) underscore the pressing need to develop approaches for delineating the sequences and functionalities of the serum antibodies elicited by vaccination (16).
Single-cell cloning has been used to identify neutralizing antibodies encoded by mBCs or plasmablasts in peripheral blood (17). However, although extremely useful for understanding of the structural mechanisms that can lead to the blockade of pathogen infection, the interrogation of single peripheral B cells alone cannot provide information on whether antibodies encoded by single B cells are also produced as secreted IgGs from BM LLPCs, and hence whether they contribute to the serological memory induced by vaccination. A detailed understanding of the diversity of serum antibodies elicited by vaccination, their functionality (e.g., antigen affinity, epitope specificity), and their relative concentrations in the blood can provide key insights toward vaccine evaluation and development.
Here, we deployed high-resolution liquid chromatography (LC) tandem MS (MS/MS) (18–20) for the molecular-level analysis of the serum IgG repertoire, combined with deep sequencing of the V gene repertoire of peripheral B lymphocyte subsets (20) and subsequent expression and characterization of representative serum antibodies, to map the dynamics of the human humoral response to vaccination in unprecedented detail. We elected to analyze the response to booster immunization of the TT vaccine because (i) it elicits a highly effective neutralizing response that is protective toward Clostridium tetani challenge; (ii) the vaccine is highly efficacious, and as a result, no deaths from tetanus intoxication have been reported in the United States for individuals who have completed at least primary immunization (21); (iii) TT has been used as a model for analyzing B-cell development following vaccination in humans (6, 22, 23); and (iv) although early serological and mAb studies had pointed to the C-terminal fragment of the toxin heavy chain [recombinant TT fragment C (rTT.C)] as the target for antibody-mediated protection (24), the precise mechanism by which antibodies elicited by the vaccine mediate neutralization has remained unclear.
We show that the anti-TT serum IgG repertoire at steady state is composed of a limited number of antibody clonotypes (∼80–100) displaying uniformly high antigen affinity (low nanomolar or subnanomolar), that most of the serum repertoire postboost comprises preexisting (i.e., prevaccination) serum antibody clonotypes, and that there is only partial overlap between the peak-wave plasmablast V gene repertoire and the TT+ serum IgG repertoire at steady state after vaccination. We identified several serum monoclonal IgGs that bind to rTT.C, and epitope mapping revealed that all rTT.C-specific antibodies tested bind to an immunodominant linear epitope at the ganglioside-binding site of the toxin that is used for cell entry. Computational antibody docking substantiated that binding of these antibodies to the toxin blocks access to the ganglioside ligand, thus providing a possible mechanistic explanation for how the TT vaccine confers protection. These results highlight the importance of understanding the composition and dynamics of the serum antibody repertoire, together with the V gene repertoire in peripheral B lymphocytes, for the molecular understanding of vaccine function.
Results
Sequencing of the Peripheral B-Cell Repertoire After TT Booster Vaccination.
The inactivated TT vaccine is highly effective (21, 25), eliciting antibody titers in adults between 0.05 and 39.6 IU/mL, with a mean antibody titer of 1.2 IU/mL (20 μg/mL) (26). The TT-specific antibody titer decays linearly with a t1/2 of 11 y (10); therefore, booster vaccinations are recommended every 10 y. Two healthy donors (HDs) were administered the TT/diphtheria toxoid (DT) vaccine 7 y and 10 y (HD1 and HD2, respectively) following the last booster vaccination. As expected, a rapid expansion of TT+ plasmablasts (CD3−CD14−CD19+CD27++CD38++CD20−TT+) was observed at day 7 (Fig. S1). This wave of day 7 antigen-specific plasmablasts represents a transient population of responding B cells, with <30 TT+ plasmablasts per milliliter detected at t = day 0 or at t = day 56 and beyond. The peripheral blood concentration of TT-specific mBCs remained relatively constant from t = 40 d to t = 169 d (Fig. S1). The VH repertoires for each donor, encoded by day 7 plasmablasts and by IgD− mBCs collected on both day 7 and 3 mo postboost, were determined by 454 (Roche Diagnostics GmbH) sequencing (70,326 and 157,089 high-quality VH reads for HD1 and HD2, respectively; Table S1) and indexed by their VH clonotype. The VH clonotype, which represents a cluster of antibodies that likely originate from a single B-cell lineage (27, 28), is defined here as the group of VH sequences that share germ-line V and J segments and also exhibit greater than 90% amino acid identity in the complementarity-determining region (CDR)-H3 (threshold for CDR-H3 amino acid identity determined by analysis of test sets from clustered deep-sequencing data; Fig. S2). We observed that the day 7 TT+ plasmablast samples comprised 922 and 538 VH clonotypes for HD1 and HD2, respectively.
Serum Proteomics of the TT-Specific IgG Repertoire.
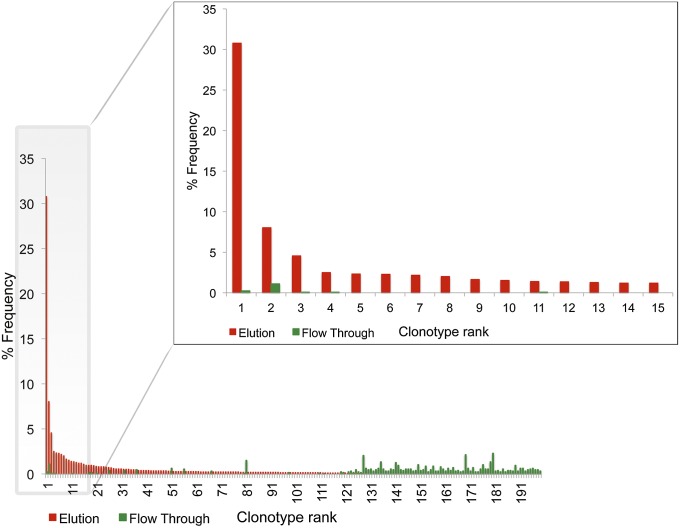
The TT+ serum IgG repertoires at t = day 0, t = 7 d, t = 3 mo, and t = 9 mo postboost were analyzed using recently developed LC-MS/MS proteomic methodology (20). Importantly, in F(ab′)2 resulting from trypsin digestion of IgG, the presence of a conserved cleavage site (Arg) directly upstream of the CDR-H3 and at the fourth residue of the downstream CH1 constant region (Lys) consistently yields a peptide encompassing the highly informative CDR-H3 and the J region (Fig. S3). Proteolysis of the F(ab′)2 with other selective proteases (e.g., GluC/LysC) resulted in peptide identifications of very few additional clonotypes (<8% additional high-confidence identifications of those found in trypsinized sample for HD2 at day 0), the vast majority of which were of low abundance. For peptide identifications, a custom database of the antibody repertoire was built using high-quality V gene sequences from the peripheral B cells in each donor (Table S1), in conjunction with a standard shotgun proteomic pipeline with a high-mass accuracy filter (average mass deviation <1.5 ppm) to minimize false identifications (20). Frequencies of antigen affinity chromatography elution- and flow-through–derived CDR-H3 peptides mapping to a unique clonotype in the 454 donor-specific sequence database are shown in Fig. 1. The serum IgG clonotype frequency histograms are highly reproducible among technical replicates (20).
Fig. 1.
Representative histogram of antibody clonotype frequencies identified proteomically in the F(ab′)2 elution and flow-through fractions following TT affinity purification. The histogram shown depicts the 3-mo postboost serum IgG repertoire for HD1. Frequencies shown here were calculated by adding the CDR-H3 spectral counts for all peptides mapping to a single clonotype.
Sensitivity and Resolution of CDR-H3 Peptide Quantitation.
To determine the dynamic range of detection of serum antibodies and to calibrate the resolution of antibody quantitation, isotopically labeled peptides corresponding to seven TT-specific CDR-H3 sequences observed over a wide range of MS peak intensities in serum samples from donor HD1 and ranging from 15 to 25 residues in length (i.e., largely spanning the observed CDR-H3 peptide length distribution) were synthesized and spiked into trypsinized HD1 samples at varying amounts (5–500 fmol). For all seven synthetic peptides, peak intensities varied linearly with peptide concentration (Spearman correlation = 0.98) and displayed small differences (less than threefold) across different peptides at each spike-in concentration (Fig. S4). The LC-MS/MS detection limit was found to be 5 fmol. Thus, based on the amount of trypsinized F(ab′)2 injected, we estimate the lower limit of sensitivity of IgG in the serum at ∼0.1 nM (or ∼15–16 ng/mL).
Identities and Dynamics of the Serum Antibody Response to Vaccination.
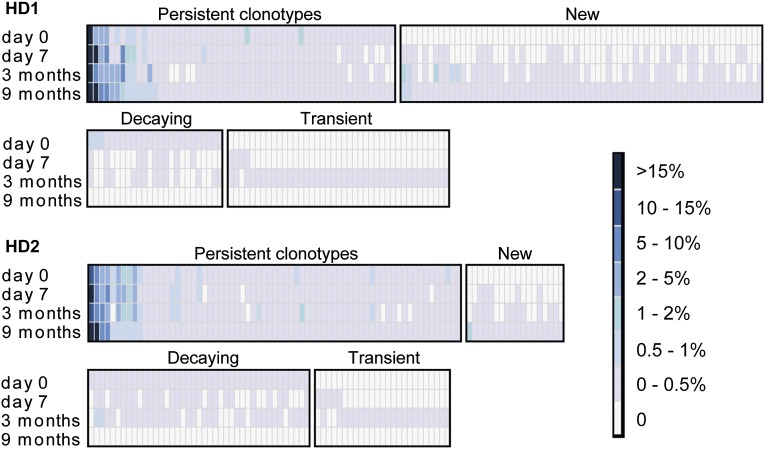
The composition, persistence, and dynamics of VH clonotype frequencies in the TT-specific serum IgG repertoire at t = day 0, t = day 7, t = 3 mo, and t = 9 mo postboost are shown in Fig. 2; the t = day 0 and t = 9 mo time points constitute the steady-state response pre- and postboost vaccination. At steady state pre- and postboost, the TT+ serum repertoire displays a comparable clonal diversity in both donors, comprising between 82 and 124 antibody clonotypes. Particularly striking features of the serological repertoire include the following:
-
i)
For both donors at 9 mo postboost, >40% of the detectable serum antibody response (the sum of all TT+ CDR-H3 peptide peak intensities) could be traced to three antibody clonotypes. The vast majority of the rest of the TT-specific clonotypes were present at frequencies under 0.5%. Specifically, in both donors, we estimate that the most abundant TT-specific antibody clonotype is present in the serum at a concentration of several micrograms per milliliter (∼15–20 μg/mL based on the MS peptide peak intensity and peptide concentration calibration in Fig. S4).
-
ii)
In both HD1 and HD2, highly abundant serum antibody clonotypes at day 0 were also present at high concentrations 9 mo postboost.
-
iii)
A transient increase in the TT+ serum IgG clonotypic diversity index (IgG-cd index) was observed at 3 mo postvaccination (82% increase in the IgG cd-index for HD1 and 17% increase for HD2); the smaller increase in the IgG-cd index for HD2 may be related to the fact that this donor did not display a higher TT titer postvaccination (Table S2) even though a robust cellular response to the vaccine was evident at day 7 (Fig. S1).
-
iv)
The postboost steady-state repertoire comprised 59% (HD1) and 21% (HD2) new TT+ antibody clonotypes not observed in the t = day 0 sample. The overwhelming majority of these were detected at low levels, with only one to two new antibody clonotypes in each donor detected at levels ≥0.5% of the antigen-specific serum repertoire.
Fig. 2.
Heat map of TT+ serum IgG clonotypes. Binned frequencies of TT+ serum clonotypes are determined from mass spectral peak intensities [extracted-ion chromatogram (XIC) peak areas] of CDR-H3 peptides. Each column represents a unique clonotype across four different time points relative to the booster vaccination. Persistent clonotypes (present at day 0 and 9 mo) and new clonotypes (not detected at day 0 yet detected at 9 mo) are ordered by the postboost steady-state (9 mo) frequency. Decaying clonotypes (present at day 0 but not detected at 9 mo) are ordered by the preboost steady-state (day 0) frequency. Transient clonotypes are not detected at pre- or postboost steady state.
Comparison of the Peripheral B-Cell and TT+ Serum Antibody Clonotypes.
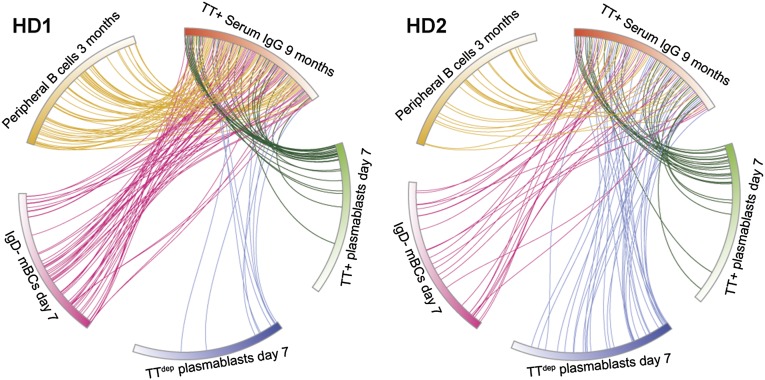
Earlier studies in mice had revealed a wide disparity in clonal diversity between the antigen-specific peripheral B cells and the steady-state LLPC population in the BM that is predominantly responsible for the synthesis of the antibodies that constitute the serological memory (29). It had been estimated that only ∼10% of responding peak plasmablasts following TT boost vaccination in humans differentiate further into LLPCs (30). Consistent with these observations, comparison of the steady-state TT+ serum IgG repertoire with the repertoire encoded by the day 7 TT+ plasmablasts revealed that <5% of the peak-wave (day 7) TT-specific plasmablast clonotypes contribute to the serological memory at a level sufficiently high enough to be detected (Fig. 3 and Table S1). Additionally, a small percentage (<1%) of the day 7 plasmablast clonotypes that did not stain with fluorescently labeled TT [i.e., TT depleted (TTdep)] encode antibodies that were detected in the TT+ serum IgG repertoire. These TTdep plasmablasts likely expressed insufficient levels of membrane IgG to stain with TT during fluorescence-activated cell sorting (FACS) yet encoded TT-specific antibodies, and consequently ended up contributing to the serological memory.
Fig. 3.
Circos plots show the relationship between the B-cell and TT+ serum IgG repertoire. Postboost steady-state (9 mo) TT+ serum IgG clonotypes (red section, ordered clockwise by frequency) and the V gene repertoire clonotypes determined by 454 sequencing of each B-cell subset, ordered clockwise by frequency, in HD1 (Left) and HD2 (Right) are shown. IgD− mBC day 7: CD3−CD14−CD19+CD27+CD20+IgD−, TT+ plasmablasts day 7: CD3−CD14−CD19+CD27++CD38++CD20−TT+, TTdep plasmablasts day 7: CD3−CD14−CD19+CD27++CD38++CD20−. Even though some low-abundance TT+ serum IgG clonotypes solely map to day 7 TTdep plasmablasts, these cells did not stain with antigen by FACS, likely due to varying levels of B-cell receptor expression and affinities for the antigen-dye conjugate. Peripheral B cells 3 mo: combined IgD− mBCs and total plasmablast cells. Clonotype numbers for each population are provided in Table S1.
Many highly expanded clonotypes encoded by the day 7 TT+ plasmablasts (as determined by high 454 sequencing read frequencies) were also detected at high or moderately high levels in the serum at 9 mo (Fig. 3). This indicates, albeit not directly, that these respective TT+ plasmablasts likely developed into LLPCs at steady state after vaccination. Direct observation of these LLPCs over the course of a vaccine response is extremely difficult in humans, because most LLPCs reside in the BM. In both donors, there were, however, several highly expanded day 7 TT+ plasmablast clonotypes that encoded antibodies not detected in the 9-mo serological repertoire. To examine whether the absence of these antibodies in the serum was due to a technical inability to detect the respective tryptic peptide fragments by MS, we selected one of the antibodies found at a very high 454 sequencing read frequency (9%) in the day 7 TT+ plasmablast repertoire from HD2, yet absent from the serological repertoire at all time points, and expressed it in HEK 293F cells. We found that the CDR-H3 tryptic peptides of this recombinant antibody were readily observable by LC-MS/MS (Fig. S5). This result strongly suggests that the TT+ plasmablast clonotypes that could not be detected by our proteomic analysis are either not present in the serum at all or are present at subphysiological concentrations, below the 0.1 nM LC-MS/MS detection limit.
Characterization of TT-Specific Antibodies.
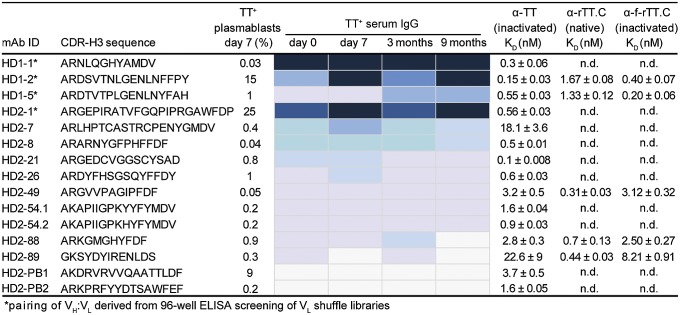
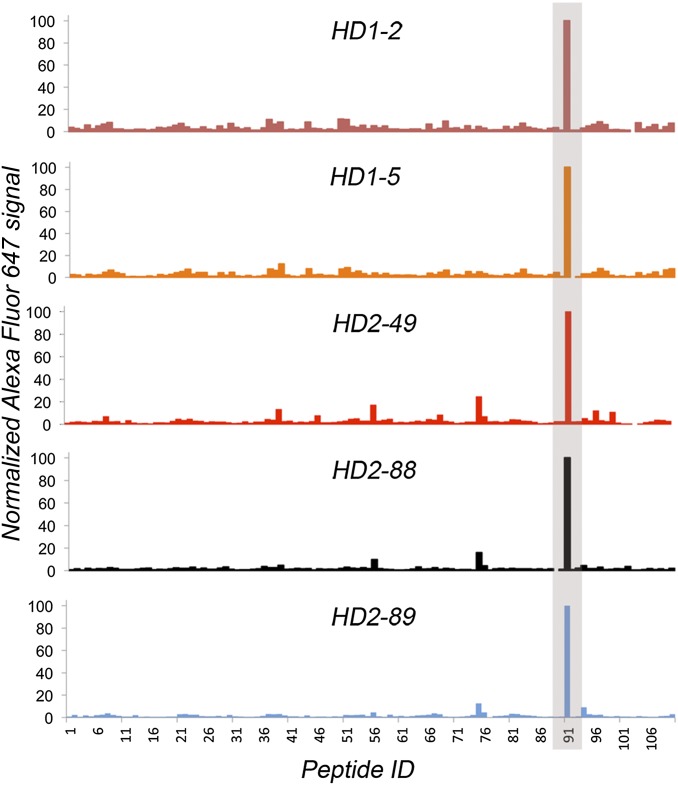
VH/VL pairs encoded by TT+ plasmablasts from HD2 were determined by a high-throughput method (19), and nine of those for which the clonotype was detected in the TT+ serum IgG repertoire were expressed as human IgG1 antibodies in HEK 293F cells. These experiments used previously frozen plasma cells, which are known to display low viability upon thawing; consequently, some VH/VL pairs of particular interest could not be determined. Therefore, for the highest frequency TT+ serum clonotype from HD2 and three high-frequency TT+ serum clonotypes from HD1, VH genes were first synthesized and used to construct four separate Fab libraries in bacteria with VL cDNA from donor-specific TT+ plasmablasts (20). Because of the low diversity of the TT+ plasmablast-derived VL pool (1,100–1,300 unique CDR-L3 genes), these libraries could be readily screened by ELISA, resulting in the isolation of productive VH/VL pairs for each clonotype (Fig. S6). Overall, we determined the affinity (Kd) of 13 full-length serum IgGs to vaccine-grade TT (formaldehyde-inactivated), to native rTT.C, and to formaldehyde-treated rTT.C (Fig. 4). As expected for booster vaccination, all antibodies bound the vaccine antigen TT with high affinities (Kd ∼ 2.2 × 10−8 − 1 × 10−10 M). Neutralizing epitopes of the 150-kDa holotoxin have been reported to reside within rTT.C (24). Five serum IgGs (HD1-2, HD1-5, HD2-49, HD2-88, and HD2-89) were shown to bind to rTT.C with subnanomolar Kd values. Epitope binding analysis using an overlapping 15-mer peptide array spanning the entire rTT.C polypeptide (110 peptides in the array) revealed that all five rTT.C-specific antibodies recognized a single linear epitope (Fig. 5). Modeling of the Ab/Ag complex of HD2-49, HD2-88, and HD2-89 (Fig. S7) using Rosetta software showed that binding of these antibodies to rTT.C occludes the ganglioside-binding site, which serves as the cell surface ligand used by the toxin for its internalization, and thus may play a critical role in toxin neutralization. The structural models reveal numerous contacts between the antibody CDR-H3 and CDR-L3 loops and the experimentally defined binding epitope of the toxin.
Fig. 4.
Functional analysis of recombinant TT-specific mAbs from serum. VH/VL pairs from day 7 TT+ plasmablasts were determined (19), genes were synthesized, and IgG antibodies were expressed in HEK 293F cells. For the four IgGs marked with an asterisk, the VL genes were identified by screening libraries of each of the respective synthetic VH genes paired with TT+ plasmablast VL repertoires on 96-well ELISA plates. The mAb ID indicates the donor from which the antibodies were derived, followed by the serum IgG clonotype ranking (by postboost steady-state frequency). For each VH, the respective clonotype frequency (%) in the day 7 TT+ plasmablast V gene repertoire is shown. The heat map indicates the relative frequency of the clonotype for each VH (a heat map key is provided in Fig. 2). Equilibrium dissociation constants (Kd values) toward vaccine-grade TT (TT), native rTT.C, and formaldehyde-inactivated rTT.C (f-rTT.C) were determined by competitive ELISA. HD2-PB1 and HD2-PB2 correspond to VH genes found in the day 7 TT+ plasmablast VH gene repertoire but not detected in the serum at any time point examined. MS analysis of trypsin digests of recombinant HD2-88 and HD2-PB1 is illustrated in Fig. S5. Full amino acid sequences of analyzed TT-specific mAbs are provided in Fig. S6. n.d., not determined.
Fig. 5.
Epitope mapping of rTT.C-specific antibodies. The histogram summarizes the normalized signal of five rTT.C-specific antibodies binding to a peptide array scan of an rTT.C sequence. The peptide array contained 110 15-mer peptides having a 12-residue overlap and spanning the entire rTT.C sequence. A negative control using Herceptin resulted in very low signal across the peptide array and positive control spots (absorbed full-length TT and rTT.C proteins). Computational modeling of three of these antibodies with the experimentally defined epitope is shown in Fig. S7.
Discussion
The majority of approved and experimental vaccines elicit long-lasting humoral immunity and a protective response that manifests as a significant titer of neutralizing serum antibodies (2). Vaccine development and evaluation have so far relied on the determination of neutralization titers, with titers higher than a certain threshold deemed protective. However, antibody titers constitute an aggregate property from which it is very difficult to infer, let alone to ascertain, the precise mechanism of protection conferred by the multiplicity of antibodies contained in the serum (31). Additionally, many experimental vaccines fail because they cannot elicit prolonged neutralizing antibody responses or because the vaccine response is directed toward nonneutralizing or heterologous epitopes (15); thus, it is important to be able to trace the identities, dynamics, and binding functionality of the monoclonal serum antibodies induced by vaccination to identify B cells encoding these antibodies, and to delineate their developmental trajectory and fate.
We have developed a platform technology for determining both the serological antibody repertoire and the V gene repertoires in peripheral B cells. Combined with expression of representative antibodies found in serum, antigen affinity and epitope mapping studies, and Rosetta modeling of antibody–antigen complexes, we show here that this pipeline can provide completely unique insights on the nature of humoral responses elicited by vaccination through a direct proxy of the serum antibody vaccine response. In two donors, we find that the anti-TT polyclonal response comprised approximately the same number of distinct antibody clonotypes at steady state (IgG-cd index = 80–120). As expected, a transient increase in the serological clonotypic diversity was observed after the peak response and was most pronounced at 3 mo after vaccination. For comparison, the serum of hyperimmunized rabbits exhibited a more restricted diversity of antigen-specific antibodies (IgG-cd index = 30) (20).
We note that the estimation of the IgG-cd index is subject to the established limitations of LC-MS/MS proteomic analysis and likely represents a lower estimate of the true diversity of the serological repertoire, because some CDR-H3–derived peptides are present below the level of detection. Nevertheless, several lines of evidence indicate that the LC-MS/MS serum proteomics we used here clearly capture the salient features of the serological repertoire. First, digestion of serum antibodies with proteases other than trypsin resulted in identification of only a small number of additional low-frequency antibody clonotypes. Second, analysis of the V gene repertoire suggests that tryptic digestion suffices to generate peptide fragments of appropriate size for MS analysis for >92% of the V gene sequences in the 454 database (Fig. S3). Third, because of the presence of conserved trypsin cleavage sites adjacent to the CDR-H3/J region, the CDR-H3–derived peptides contain the highly conserved J region, and thus demonstrate similar characteristics and MS observability (Fig. S5). The similarity in the MS observability of CDR-H3–derived tryptic peptides therefore facilitates their relative quantitation by measuring peak intensities. Finally, with a detection limit of 0.1 nM, LC-MS/MS serum proteomics are easily able to identify antibodies that are present at physiologically relevant concentrations (i.e., concentrations >Kd), given that the theoretical ceiling of antibody affinity is 0.1 nM and the overwhelming majority of antibodies are expected to display higher Kd values, typically in the nanomolar range. For these reasons, the IgG-cd index provides a useful metric for evaluating differences in the breadth of the polyclonal vaccine response across samples.
Upon restimulation with antigen, mBCs undergo rapid expansion and differentiation into plasmablasts that emigrate into peripheral blood. In humans, the number of plasmablasts in blood reaches a peak value 7 d after immunization and then declines rapidly (32). Although the large majority of plasmablasts in the periphery undergo apoptosis, a small fraction, estimated at between 10% and 20% (30), are thought to be able to home into the BM, where they survive for extensive periods of time as LLPCs, producing the antibodies that constitute the long-term serological memory. Comparison of the plasmablast clonotypic repertoire with the anti-TT serum IgG repertoire revealed that ∼5% of the peak TT+ plasmablast clonotypes contribute to the steady state (long-term) serological memory. These “successful” TT+ plasmablast clonotypes were mostly present at high frequencies, cumulatively 38% and 30% (HD1 and HD2, respectively) of the total 454 reads in the day 7 TT+ plasmablast population. Still, the data in Fig. 3 reveal that there are a significant number of the responding TT+ plasmablast clonotypes that do not contribute significantly to the long-term serological memory, suggesting that these plasmablasts are mostly short-lived populations of B cells. The cellular features that render some highly expanded plasmablast clonotypes capable of producing antibody at a steady state, whereas others do not, will need to be defined further.
We observed a striking polarization of the serological repertoire, with a dominant antibody clonotype comprising 20% of the total peptide counts detected by LC-MS/MS, and estimated it to be present at a concentration >10 μg/mL in serum. Only four to seven antibody clonotypes in every sample analyzed were estimated to be present at concentrations >1 μg/mL, whereas the majority of the antibodies in the serum repertoire were detected at lower concentrations, generally within the range of 1–4 nM. The level of polarization seen here in the serum antibody repertoire, with a handful of serum antibody clonotypes dominating the response, displays some similarities to the peripheral B-cell repertoire determined by next-generation sequencing following flu immunization (33). Further, some serum antibodies (e.g., HD2-7) appear to be present in the serum at a concentration below the Kd value (Fig. 4, Kd for formaldehyde-treated TT =18.6 nM compared with an estimated serum concentration of 5 nM). Upon challenge with TT, such antibodies would be expected to be largely in the unbound state at equilibrium, and thus may not contribute significantly to protection.
Proteomic serum antibody profiling of the vaccine response at a molecular level facilitates a repertoire-wide analysis of neutralizing functionality of the constituent antibodies, which presents obvious utility in probing the efficacy of a vaccine in eliciting targeted responses toward neutralizing epitopes. For both donors, we find that recombinant IgGs encoded by the dominant clonotype in HD1 and HD2 bind to formaldehyde-treated TT with subnanomolar affinity; however, because they do not recognize the rTT.C fragment of the toxoid, they are unlikely to play an important role in neutralization despite their presence at high concentrations. Likewise, the majority (eight of 13) of serum antibodies tested also did not recognize rTT.C. In HD1, two of the top most abundant antibodies examined, HD1-2 and HD1-5 (observed at an estimated serum concentration of 10 μg/mL and 2 μg/mL, respectively) recognized native rTT.C protein with high affinity. Remarkably, epitope mapping revealed that both HD1-2 and HD1-5, as well as all serum anti-rTT.C IgGs identified in HD2, recognize the same linear epitope. Rosetta docking further showed that antibody binding to rTT.C occludes the ganglioside-binding site used by the toxin to gain entry into cells. These findings reveal that the existence of an immunogenic epitope at the ganglioside-binding site of the TT plays an important role in the elicitation of antibodies with neutralizing potential, and thus provides a molecular-level explanation of the action of the vaccine. Molecular-level identification and subsequent analysis of serum antibody-binding functionality provide obvious utility in understanding the effectiveness of a vaccine in eliciting neutralizing vs. nonneutralizing antibodies, as well as in understanding the effect of this balance on vaccine outcome (15).
Materials and Methods
Vaccination and Titers.
A healthy male and female donor each received a booster vaccination comprising TT/DT [20 international units (IU) TT and 2 IU DT; Sanofi Pasteur MSD GmbH] after informed consent had been obtained. Serum anti-TT titers were determined in triplicate on ELISA plates coated with 2 μg/mL purified vaccine-grade TT (Statens Serum Institut). The anti-TT World Health Organization International Standard for Tetanus Immunoglobulin, Human (National Institute for Biological Standards and Control code TE-3) was included in triplicate to allow conversion to international units per milliliter.
High-Throughput Sequencing of VH and VL Repertoires.
Peripheral blood mononuclear cells were isolated and stained for FACS sorting as described in SI Materials and Methods. For each FACS-sorted B-cell population, first-strand cDNA was generated from total RNA using a SuperScript RT II kit (Invitrogen) and oligo-dT primer. Vλ, Vκ, and VH repertoires were PCR-amplified as described (34). These amplified V gene repertoires from each sorted B-cell population were sequenced using high-throughput 454 GS-FLX sequencing (University of Texas at Austin and SeqWright). Raw 454 fasta files were submitted to the international ImMunoGeneTics database High V-Quest for V gene sequence alignment (35). Unique, full-length VH gene sequences for each donor were assigned into clonotypes as described above.
Proteomic Analysis of the Serum Antibodies to TT in Human Donors.
For each sample, IgG was purified from 7 to 9 mL of serum by protein G enrichment, followed by pepsin digestion to generate F(ab′)2 and Fc fragments. Antigen-specific F(ab′)2 fragments were isolated by TT-affinity column chromatography. Elution and flow-through fractions were trypsin-digested, and resulting peptides were fractionated and sequenced by nanoflow LC-electrospray MS/MS on an Orbitrap Velos Pro hybrid mass spectrometer (Thermo Scientific). The resulting spectra were searched against a custom protein sequence database as described (20) and detailed in SI Materials and Methods. The IgG-cd index represents the total number of serum IgG clonotypes detected in the serum of an individual at a distinct time point. Absolute peptide quantitation was done using isotopically labeled peptides spiked into MS-ready donor samples at different stoichiometric amounts and searched against the donor sequence database with isotopic labels included as dynamic modifications in the search.
Construction and Characterization of Recombinant IgG.
VH/VL pairing of HD2 day 7 TT+ plasmablasts was carried out as described (19). In addition, synthetic genes encoding the high-frequency, proteomically identified VH sequences were paired with the VL repertoire from day 7 TT+ plasmablasts, expressed as Fabs in Escherichia coli, and screened for TT specificity by ELISA. VH/VL pairs exhibiting high ELISA signal were cloned and purified as IgG from HEK 293F cells. IgG affinities for vaccine-grade TT, rTT.C, and formaldehyde-inactivated rTT.C were determined by competitive ELISA.
Peptide Microarrays.
Pepstar peptide microarrays (JPT Peptide Technologies GmbH) were constructed from overlapping 15-mer peptides (12-aa overlap) derived from rTT.C. Each microarray included three identical subarrays as technical replicates. Control rTT.C and TT spots were at separate locations in the array. The binding of purified recombinant antibodies to the peptide array was carried out according to the manufacturer’s instructions, with modifications detailed in SI Materials and Methods.
Antibody Homology Modeling and Clustering Methods.
Antibody VH and VL structures were determined by structural homology modeling using the Rosetta 3.5 suite of protein structure prediction and design software (36). Antibody homology models were then docked to rTT.C (Protein Data Bank ID code 1AF9) and analyzed as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Scott Hunicke-Smith for assistance with next-generation sequencing, Constantine Chrysostomou for assistance in data analysis, Chhaya Das for recombinant IgG expression, Prof. Jeffrey J. Gray and Dr. Daisuke Kuroda for advice and assistance on the Rosetta modeling, Alexa Rodin for assistance with experiments, T. Kaiser and J. Kirsch for assistance with cell sorting, and Prof. Brent L. Iverson for useful discussion and comments. Funding for this work was provided by the Clayton Foundation (G.G.), by Welch Foundation Grant F1515 (to E. M. Marcotte), by the Defense Advanced Research Projects Agency (G.G. and A.D.E.), and by National Institutes of Health (NIH) Grants 5 RC1DA028779 (to G.G.) and GM 076536 (to E. M. Marcotte). J.J.L. was supported by a postdoctoral fellowship from the Cancer Prevention Research Institute of Texas. The LTQ Orbitrap Velos Pro instrument was purchased with support by the NIH Western Research Center of Excellent in Biodefense (NIH Grant 5U54AI057156) and the Texas Institute for Drug and Diagnostics Development (Grant TI-3D).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317793111/-/DCSupplemental.
References
- 1.Germain RN. Vaccines and the future of human immunology. Immunity. 2010;33(4):441–450. doi: 10.1016/j.immuni.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rappuoli R. Bridging the knowledge gaps in vaccine design. Nat Biotechnol. 2007;25(12):1361–1366. doi: 10.1038/nbt1207-1361. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 6.Frölich D, et al. Secondary immunization generates clonally related antigen-specific plasma cells and memory B cells. J Immunol. 2010;185(5):3103–3110. doi: 10.4049/jimmunol.1000911. [DOI] [PubMed] [Google Scholar]
- 7.Mei HE, et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood. 2009;113(11):2461–2469. doi: 10.1182/blood-2008-04-153544. [DOI] [PubMed] [Google Scholar]
- 8.Reddy ST, et al. Monoclonal antibodies isolated without screening by analyzing the variable-gene repertoire of plasma cells. Nat Biotechnol. 2010;28(9):965–969. doi: 10.1038/nbt.1673. [DOI] [PubMed] [Google Scholar]
- 9.Benner R, Hijmans W, Haaijman JJ. The bone marrow: The major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981;46(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 12.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol. 2012;12(1):24–34. doi: 10.1038/nri3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331(6021):1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radbruch A, et al. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6(10):741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 15.Khurana S, et al. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med. 2013;5(200):200ra214. doi: 10.1126/scitranslmed.3006366. [DOI] [PubMed] [Google Scholar]
- 16.Crowe JE. Universal flu vaccines: Primum non nocere. Sci Transl Med. 2013;5(200):200fs234. doi: 10.1126/scitranslmed.3007118. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PC, Andrews SF. Tools to therapeutically harness the human antibody response. Nat Rev Immunol. 2012;12(10):709–719. doi: 10.1038/nri3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung WC, et al. A proteomics approach for the identification and cloning of monoclonal antibodies from serum. Nat Biotechnol. 2012;30(5):447–452. doi: 10.1038/nbt.2167. [DOI] [PubMed] [Google Scholar]
- 19.DeKosky BJ, et al. High-throughput sequencing of the paired human immunoglobulin heavy and light chain repertoire. Nat Biotechnol. 2013;31(2):166–169. doi: 10.1038/nbt.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wine Y, et al. Molecular deconvolution of the monoclonal antibodies that comprise the polyclonal serum response. Proc Natl Acad Sci USA. 2013;110(8):2993–2998. doi: 10.1073/pnas.1213737110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ataro P, Mushatt D, Ahsan S. Tetanus: A review. South Med J. 2011;104(8):613–617. doi: 10.1097/SMJ.0b013e318224006d. [DOI] [PubMed] [Google Scholar]
- 22.Franz B, May KF, Jr, Dranoff G, Wucherpfennig K. Ex vivo characterization and isolation of rare memory B cells with antigen tetramers. Blood. 2011;118(2):348–357. doi: 10.1182/blood-2011-03-341917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-García I, Rodríguez-Bayona B, Mora-López F, Campos-Caro A, Brieva JA. Increased survival is a selective feature of human circulating antigen-induced plasma cells synthesizing high-affinity antibodies. Blood. 2008;111(2):741–749. doi: 10.1182/blood-2007-08-108118. [DOI] [PubMed] [Google Scholar]
- 24.Fotinou C, et al. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J Biol Chem. 2001;276(34):32274–32281. doi: 10.1074/jbc.M103285200. [DOI] [PubMed] [Google Scholar]
- 25.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schauer U, et al. Levels of antibodies specific to tetanus toxoid, Haemophilus influenzae type b, and pneumococcal capsular polysaccharide in healthy children and adults. Clin Diagn Lab Immunol. 2003;10(2):202–207. doi: 10.1128/CDLI.10.2.202-207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moody MA, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS ONE. 2011;6(10):e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulsen TR, Jensen A, Haurum JS, Andersen PS. Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J Immunol. 2011;187(8):4229–4235. doi: 10.4049/jimmunol.1000928. [DOI] [PubMed] [Google Scholar]
- 29.Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med. 2011;208(13):2599–2606. doi: 10.1084/jem.20110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Höfer T, et al. Adaptation of humoral memory. Immunol Rev. 2006;211:295–302. doi: 10.1111/j.0105-2896.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 31.Georgiev IS, et al. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science. 2013;340(6133):751–756. doi: 10.1126/science.1233989. [DOI] [PubMed] [Google Scholar]
- 32.Odendahl M, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105(4):1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5(171):171ra19. doi: 10.1126/scitranslmed.3004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ippolito GC, et al. Antibody repertoires in humanized NOD-scid-IL2Rγ(null) mice and human B cells reveals human-like diversification and tolerance checkpoints in the mouse. PLoS ONE. 2012;7(4):e35497. doi: 10.1371/journal.pone.0035497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefranc MP, et al. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 1999;27(1):209–212. doi: 10.1093/nar/27.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann KW, Lemmon GH, Deluca SL, Sheehan JH, Meiler J. Practically useful: What the Rosetta protein modeling suite can do for you. Biochemistry. 2010;49(14):2987–2998. doi: 10.1021/bi902153g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.