Fig. 4.
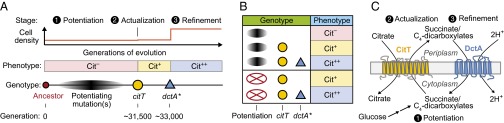
Model for the evolution of citrate utilization in the E. coli long-term evolution experiment. (A) There were three major epochs in the evolution of this metabolic innovation: potentiation, actualization, and refinement. Weakly Cit+ cells were first isolated from the population after the rnk–citG actualizing mutation that amplified and activated the CitT transporter (shown as the citT allele). There was not an appreciable increase in the final cell density at the end of each growth cycle in the evolution experiment at this point (Top). After the dctA* refinement mutation, there was a substantial increase in population size because cells were able to fully use citrate, which we distinguish from the rudimentary Cit+ phenotype as the strong Cit++ phenotype (Middle). The evolution of Cit++ is statistically more likely from certain Cit– genetic backgrounds that arose later in this population, presumably because they accumulated one or more potentiating mutations relative to the ancestor (3, 10). Key mutations are shown with their approximate timings relative to these evolutionary epochs and one another (Bottom). (B) Cit++ phenotype is not the product of all-or-none epistasis with potentiating mutations. The progression of citrate utilization phenotypes as they evolved in the LTEE in strains that contained key mutations in the context of earlier evolved alleles (Upper) and qualitative phenotypes of reconstructed strains containing only key evolved alleles in the ancestral genetic background (Bottom) are shown. The evolved citT mutation alone is sufficient for detectable but extremely limited citrate utilization, as observed in early Cit+ isolates (3). The citT and dctA* mutations together are sufficient for full citrate utilization characteristic of the Cit++ phenotype, even in the absence of potentiating mutations. (C) Mechanism of Cit++ refinement. When both the CitT and DctA transporters are expressed, due to the citT and dctA* mutations, their activities can be coupled so that the proton-motive force (H+) powers reuptake of succinate or other C4-dicarboxylate substrates for continued citrate import, yielding the Cit++ phenotype. It is possible that the unknown potentiating mutations make sufficient succinate available from glucose metabolism to power limited citrate import through CitT but that this does not result in a sustainable cycle.
