Significance
Fanconi anemia (FA) is a devastating disease associated with a progressive bone marrow failure (BMF) and clonal proliferation of primitive hematopoietic cells that leads to leukemia. In an effort to understand the molecular basis of BMF and leukemogenesis in FA, we recently uncovered a unique function of proteins associated with FA in transcriptional regulation that translates into elevated levels of the signaling molecule Dickkopf-1 (DKK1). Overproduction of DKK1 has been shown to alter functions in hematopoiesis and to promote hematologic malignancies. Thus, our findings represent a crucial step in the development of strategies aimed at preventing BMF and/or clonal hematopoiesis in patients with FA.
Abstract
Fanconi anemia (FA) is an inherited bone marrow failure syndrome associated with a progressive decline in hematopoietic stem cells, developmental defects, and predisposition to cancer. These various phenotypic features imply a role of FA proteins in molecular events regulating cellular homeostasis. Interestingly, we previously found that the Fanconi C protein (FANCC) interacts with the C-terminal-binding protein-1 (CtBP1) involved in transcriptional regulation. Here we report that FANCC with CtBP1 forms a complex with β-catenin, and that β-catenin activation through glycogen synthase kinase 3β inhibition leads to FANCC nuclear accumulation and FA pathway activation, as measured by the Fanconi D2 protein (FANCD2) monoubiquitination. β-catenin and FANCC nuclear entry is defective in FA mutant cells and in cells depleted of the Fanconi A protein or FANCD2, suggesting that integrity of the FA pathway is required for FANCC nuclear activity. We also report that FANCC with CtBP1 acts as a negative regulator of Dickkopf-1 (DKK1) expression, and that a FA disease-causing mutation in FANCC abrogates this function. Our findings reveal that a defective FA pathway leads to up-regulation of DKK1, a molecule involved in hematopoietic malignancies.
Fanconi anemia (FA) is an inherited bone marrow failure (BMF) syndrome transmitted through both autosomal and X-linked modes (1, 2). FA is associated with congenital malformations, genome instability, and a predisposition to cancer. Genetic and functional complementation approaches have helped define 16 gene products that cooperate in a molecular pathway termed the FA pathway (3, 4). This FA pathway is activated in response to cellular stress that causes interruptions in the replication or transcription processes (5). Mutations in any of these FA genes cause clinical features characteristic of FA, with BMF the most prevalent manifestation.
BMF in patients with FA results from a progressive decline in hematopoietic stem cells (HSCs), suggesting that FA proteins play a role in the maintenance of these cells. The mechanism by which FA proteins or the FA pathway act in protecting these cells remains unclear, however. Findings from several groups have shown that the FA proteins act in various cellular functions, including apoptosis suppression, cytokine signaling, and transcriptional regulation (6). Indeed, we recently reported that the Fanconi C protein (FANCC) directly interacts with the C-terminal-binding protein 1 (CtBP1) involved in transcriptional regulation (7). CtBP1 and its isoforms are involved in many cellular activities, including Golgi fission and cellular division, but predominantly in transcriptional repression (8). Many known DNA-binding transcription factors mediate transcriptional repression in a CtBP1-dependent manner, including Wnt/β-catenin/T-cell factor, bone morphogenic protein 1/transforming growth factor β, and GATA factors. Analysis of CtBP1 protein complexes have revealed the presence of histone-modifying factors as well as DNA-binding transcription factors, implying that CtBP1 exerts its transcriptional regulation effects through recruitment of cofactors (8, 9).
Interaction between FANCC and CtBP1 suggests a role of FA proteins in transcriptional regulation. In fact, we previously showed that depletion of either the CtBP1 or FA proteins modulates Wnt pathway genes, of which the Dickkopf-1 gene (DKK1) is the most up-regulated gene (7). DKK1 belongs to the Dickkopf family of secreted molecules, which antagonize Wnt signaling by sequestering the Wnt receptors low-density lipoprotein receptor-related protein 5/6 and Kremen (10). DKK1 was initially characterized for its role in developmental morphogenesis of the head, eyes, limbs, and vertebrae. The absence of Dkk1 in KO mice is associated with many congenital malformations reminiscent of features of FA, including anophthalmia and polysyndactyly (11–14), whereas DKK1 up-regulation is often found in cancers, including multiple myeloma, hepatoblastoma, Wilms’ tumor, and breast, head, neck, and oral cancers (15). These types of cancers are seen in patients with FA (16). In addition, up-regulation of DKK1 has been shown to accelerate cell cycling of HSCs and to progressively diminish the regenerative function of HSCs after transplantation (17).
The fact that FA is associated with congenital abnormalities, cancers, and HSC defects (18–22) similar to those associated with deregulation of DKK1 prompted us to investigate the functional role of FANCC–CtBP1 interaction in DKK1 transcriptional regulation. Here we demonstrate that FANCC forms a complex with CtBP1 and β-catenin. Activation of β-catenin through inhibition of glycogen synthase kinase 3 beta (GSK3β) induces nuclear accumulation of FANCC similar to β-catenin, activation of the FA pathway measured by the monoubiquitnation of the Fanconi D2 protein (FANCD2), and, subsequently, transcriptional regulation of the DKK1 gene. We also demonstrate that FANCC negatively regulates DKK1 via CtBP1, and that disease-causing mutations in FANCC abrogates this function. Our findings reveal that FANCC is involved in the transcriptional regulation of DKK1.
Results
β-Catenin Activation Induces FANCC Nuclear Accumulation.
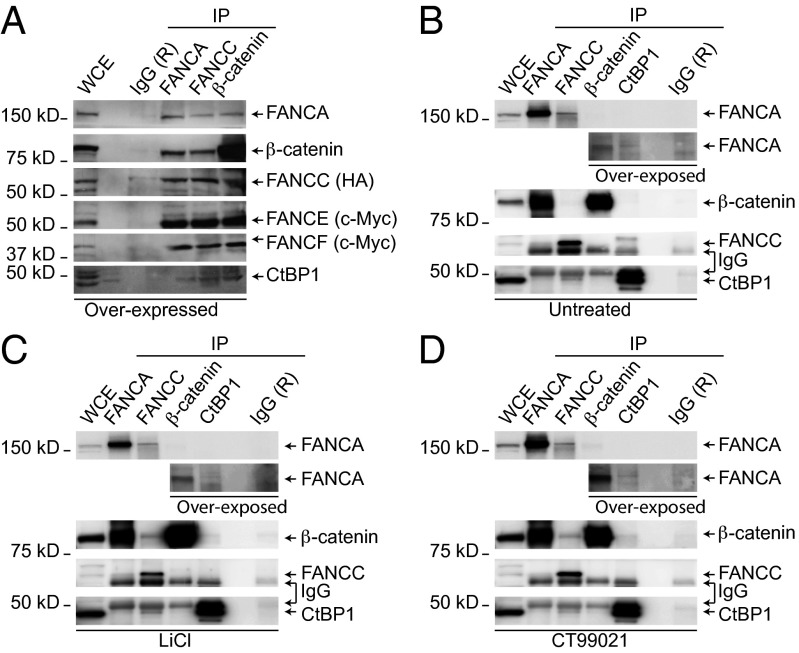
Based on our earlier observations that FANCC interacts with CtBP1 and that both proteins are involved in the regulation of the Wnt/β-catenin target genes, along with a previous study showing immunoprecipitation (IP) of β-catenin with FANCC (7, 23), we sought to determine whether FANCC with CtBP1 forms a complex with β-catenin. We performed IP experiments in human HEK293T cells expressing FANCC with β-catenin and other components of the FA core complex. Cell extracts were subjected to IP using anti-FANCA, anti-FANCC, or anti–β-catenin antibodies. Western blot analyses of the immunoprecipitates showed that the FA proteins FANCA and FANCC coimmunoprecipitated with β-catenin and endogenous CtBP1 (Fig. 1A). Western blot analysis of immunoprecipitates also showed the presence of FANCE and FANCF, suggesting that β-catenin forms a complex with FA core complex proteins.
Fig. 1.
FANCC forms a complex with CtBP1 and β-catenin. (A) HEK293T cells were cotransfected with HA-FANCC, FANCA, Myc-FANCE, Myc-FANCF, FANCG, and FANCL coding vectors. WCEs were subjected to IP with antibodies against FANCA, FANCC, or β-catenin and immunoblotted with the indicated antibodies. (B–D) WCEs from untreated HEK293T cells (B) or cells treated with the GSK3β inhibitors LiCl (C) or CT99021 (D) were subjected to IP using anti-FANCA, anti-FANCC, anti-CtBP1, or anti–β-catenin antibodies and immunoblotted with the indicated antibodies. A longer exposure time is shown below the blot (overexposed). Negative IP controls were performed using rabbit IgG (R). Representative experiments out of three total experiments are shown. Numbers indicate molecular weight.
Next, to determine whether accumulation of β-catenin favors this interaction, we performed IP studies using endogenous protein extracts from cells treated with the GSK3β inhibitors lithium chloride (LiCl) or CT99021, which are known to induce accumulation and nuclear entry of β-catenin. Our results show that treatment with GSK3β inhibitors LiCl or CT99021 induced complex formation between FANCA, FANCC, β-catenin, and CtBP1, whereas complex formation between FANCC and CtBP1 or FANCA and β-catenin occurred regardless of treatment (Fig. 1 B–D). These results suggest that FANCC in association with CtBP1 forms a complex with β-catenin after GSK3β inhibition. Taken together, these results imply that interaction between FA proteins and β-catenin occurs in the nucleus.
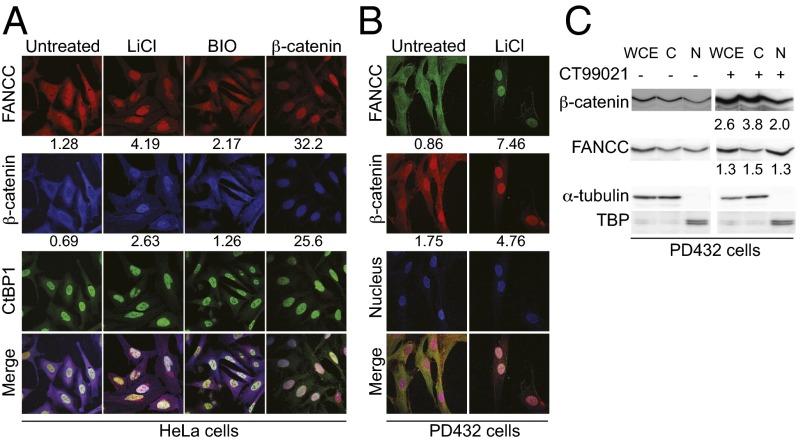
To detect the cellular localization of FANCC, CtBP1, and β-catenin, we performed immunofluorescence experiments in HeLa cells. The results showed that FANCC localized with β-catenin to the cytoplasm, whereas CtBP1 was found mainly in the nucleus (Fig. 2A). To reconcile the IP data, we performed immunofluorescence studies in cells treated with the GSK3β inhibitors LiCl or BIO/GSK3 inhibitor IX. As expected, our results show that β-catenin accumulates into the nucleus after GSK3β inhibition. Surprisingly, FANCC also accumulates into the nucleus after GSK3β inhibition, with a fourfold increase in nuclear FANCC using LiCl, a competitor for magnesium, as well as a twofold increase in nuclear FANCC using the ATP pocket inhibitor BIO/GSK3 inhibitor IX (Fig. 2A). In addition, overexpression of β-catenin, which induces its nuclear accumulation, resulted in a similar change in the localization of FANCC (Fig. 2A, fourth panel). Consequently FANCC, β-catenin, and CtBP1 localize together in the nucleus after GSK3β inhibition.
Fig. 2.
β-catenin activation induces FANCC nuclear translocation. (A) HeLa cells treated with LiCl or BIO or after β-catenin transfection were stained with anti-FANCC, anti–β-catenin, and anti-CtBP1. (B) PD432 fibroblasts treated with LiCl were stained with anti-FANCC and anti–β-catenin antibodies and TOPRO-3. Data are representative of three experiments in which at least 25 cells were analyzed at 100× magnification. Numbers indicate the mean nuclear/cytoplamic intensity ratio. (C) Western blot analysis of nuclear (N) and cytoplasmic (C) protein extracts from PD432 treated or not treated with CT99021 with the indicated antibodies. Numbers indicate the ratio of the protein detected compared with TBP (nuclear) or α-tubulin (cytoplasmic) and normalized to that of untreated cells.
We next performed similar experiments with primary fibroblast cells. Our results show that treatment of these cells with LiCl induced nuclear accumulation of β-catenin and FANCC, confirming the results observed in HeLa cells (Fig. 2B). Although nuclear staining of β-catenin appears to be elevated in untreated PD432 cells compared with HeLa cells, GSK3β inhibition induced a threefold increase in β-catenin nuclear accumulation in both cell lines. In addition, nuclear and cytoplasmic fractionation studies showed that FANCC and β-catenin protein levels increased after treatment with CT99021, with a 1.3-fold increase in nuclear FANCC and a 2-fold increase in β-catenin (Fig. 2C). These results confirm our immunofluorescence data and suggest that activation of β-catenin promotes the nuclear accumulation of FANCC.
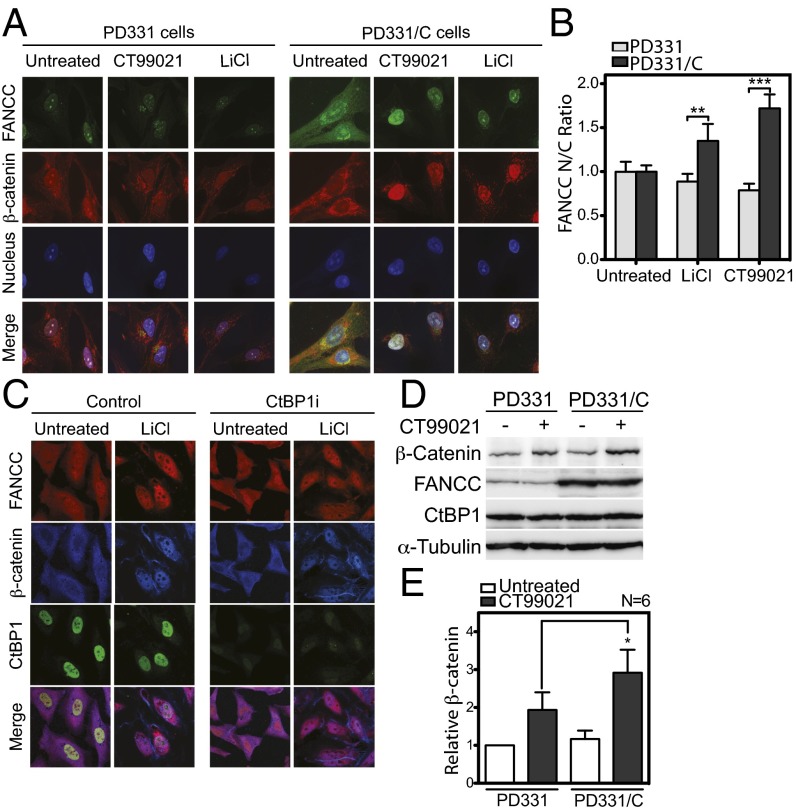
We next sought to determine whether the nuclear entry of β-catenin requires FANCC. First, we evaluated the nuclear entry of β-catenin in patient-derived FANCC-deficient cells (PD331) harboring the R548X mutation and FANCC-corrected cells (PD331/C). Cells were treated with GSK3β inhibitors LiCl or CT99021, followed by anti–β-catenin staining and visualization by confocal microscopy. We found that β-catenin failed to properly accumulate and localize to the nucleus in FANCC-deficient cells, whereas FANCC-corrected cells showed strong nuclear staining of β-catenin after GSK3β inhibition (Fig. 3A). In addition, PD331/C cells showed stronger nuclear FANCC staining after GSK3β inhibition compared with untreated cells (Fig. 3A, Right), corresponding to a twofold increase in nuclear FANCC (Fig. 3B). CtBP1 depletion did not affect the nuclear translocation of β-catenin or FANCC, as demonstrated by strong β-catenin and FANCC nuclear staining in CtBP1i and control cells transfected with nontargeting siRNA (Fig. 3C). These results suggest that FANCC, but not CtBP1, is required for efficient nuclear translocation or accumulation of β-catenin. Indeed, Western blot analyses showed that β-catenin accumulation requires FANCC, as demonstrated by the reduced β-catenin levels in patient-derived FANCC-deficient cells (PD331) after treatment with GSK3β inhibitors compared with those in FANCC-corrected cells (Fig. 3D). No significant differences in β-catenin levels were observed in untreated cells, suggesting that FANCC plays a role in β-catenin accumulation (Fig. 3E). Taken together, our results suggest that the FANCC protein plays a role in accumulation of β-catenin.
Fig. 3.
Nuclear entry of β-catenin requires FANCC. (A and B) PD331 and PD331/C cells treated with CT99021 or LiCl were stained with anti-FANCC, anti–β-catenin, and DAPI and observed at 100× magnification. (B) Data shown are mean intensity ratios of nuclear to cytoplasm FANCC representative of two experiments in which at least 25 cells were analyzed. (C) CtBP1i cells treated with LiCl were stained with anti-FANCC, anti–β-catenin, and anti-CtBP1 antibodies and were visualized at 100× magnification. (D) Western blot analysis of WCEs from PD331 and PD331/C cells treated or not treated with CT99021 with the indicated antibodies. A representative experiment out of six total experiments is shown. (E) Graph displaying the mean relative β-catenin/α-tubulin ratio ± SEM in PD331 and PD331/C cells after CT99021 treatment from six independent blots (Lower). *P ≤ 0.05.
FANCC and β-Catenin Nuclear Translocation Is Dependent on a Functional FA Pathway.
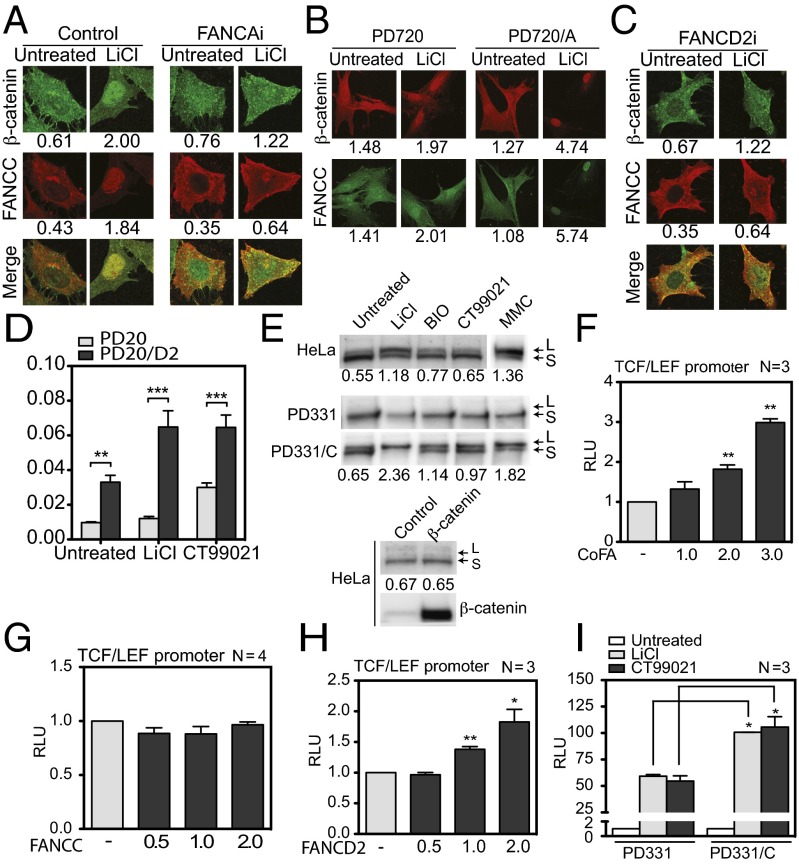
Because FANCC is a component of the FA pathway, we sought to determine whether nuclear entry of FANCC and β-catenin is dependent on a functional FA pathway. To so so, we performed immunofluorescence and cellular fractionation studies in FA-deficient cells. First, we performed immunofluorescence labeling, followed by confocal microscopy, in cells depleted of FANCA (FANCAi). In these FANCAi cells, we found that β-catenin and FANCC remained in the cytoplasm after GSK3β inhibition, in contrast to control cells transfected with nontargeting shRNA (Fig. 4A).
Fig. 4.
Nuclear translocation of β-catenin is dependent on a functional FA pathway. (A–C) FANCAi cells (A), PD720 and PD720/A fibroblasts (B), and FANCD2i cells (C) were treated with LiCl and stained with anti-β-catenin and anti-FANCC antibodies and visualized at 100× magnification. Data shown are representative of two experiments in which at least 25 cells were analyzed. Numbers indicate the mean nuclear/cytoplamic intensity ratio. (D) Graph displaying the mean relative intensity ratios of nuclear to cytoplasmic β-catenin in PD20 and PD20/D2 cells after treatment with LiCl and CT99021 from two separate experiments in which at least 25 cells were analyzed. (E) Western blot analysis of FANCD2 in WCEs from HeLa, PD331, and PD331/C cells treated or not treated with LiCl, BIO, CT99021, or mitomycin C or transfected with β-catenin or empty vector (control). (F) Luciferase assay performed in COS-1 cells transfected with the TCF/LEF reporter along with β-catenin and various concentrations of FA core complex expression vectors (FANCA, FANCC, FANCE, FANCF, FANCG, and FANCL). (G and H) Luciferase assays performed in HeLa cells transfected with the TCF/LEF reporter along with β-catenin, TCF4, and increasing amounts of FANCC (G) or FANCD2 (H) expression vectors as indicated. (I) Luciferase assays performed in PD331 and PD331/C cells transfected with the TCF/LEF reporter and exposed to LiCl or CT99021 before analysis. Numbers of experiments performed in duplicate are indicated in each graph. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
We next performed similar experiments in patient-derived FANCA-mutant cells (PD720) and FANCA-corrected cells (PD720/A). We observed that in FANCA-mutant cells, both β-catenin and FANCC are localized mainly to the cytoplasm, whereas correction of the cells with FANCA promoted a fourfold to fivefold increase in β-catenin and FANCC nuclear staining after activation of β-catenin using GSK3β inhibitors (Fig. 4B).
To determine whether the diminished nuclear entry of β-catenin is limited to cells deficient in the FA core complex proteins, including FANCA and FANCC, we used FANCD2-depleted cells (FANCD2i cells), FANCD2 being a downstream component of the FA pathway. The results indicate that β-catenin and FANCC failed to accumulate in the nucleus after GSK3β inhibition in FANCD2-depleted cells (Fig. 4C). We then performed similar experiments in patient-derived FANCD2-mutant cells (PD20) and FANCD2-corrected cells (PD20/D2). Our results show that β-catenin levels were significantly higher in PD20/D2 cells compared with noncorrected PD20 cells (Fig. 4D).
Taken together, the foregoing results suggest that nuclear accumulation of FANCC and β-catenin requires a functional FA pathway. Thus, we evaluated FA pathway activity, as measured by the monoubiquitination of FANCD2 after inhibition of GSK3β or overexpression of β-catenin. We performed Western blot analyses with protein extracts from HeLa cells treated with LiCl, BIO/GSK3 inhibitor IX, or CT99021 and with cells transfected with β-catenin. Our results show that inhibition of GSK3β, but not overexpression of β-catenin, promoted the accumulation of the monoubiquitinated form of FANCD2 (FANCD2-L) (Fig. 4E). Consistent with the role of the FA core complex in monoubiquitination of FANCD2, cells mutated in FANCC (PD331) failed to monoubiquitinate FANCD2 after GSK3β inhibition or mitomycin C treatment, whereas monoubiquitination of FANCD2 was restored in PD331/C cells.
Because the foregoing results suggest that the FA pathway may positively impact the transcriptional activity of β-catenin, we evaluated β-catenin activity using the T cell factor (TCF)/lymphoid enhancer binding factor (LEF) reporter assay. We found that overexpression of FA core-complex components increased β-catenin–mediated transcription of the TCF/LEF reporter in a dose-dependent manner (Fig. 4F). However, overexpression of FANCC alone did not influence β-catenin–mediated activation of the TCF/LEF reporter (Fig. 4G), whereas overexpression of FANCD2 increased β-catenin–mediated transcription of the TCF/LEF reporter in a dose-dependent manner (Fig. 4H). These results imply that the FA pathway acting through FANCD2 is required for efficient β-catenin activity.
To confirm these results, we used FA patient-derived mutant PD331 cells and found that these cells had significantly reduced β-catenin activity compared with FANCC-corrected cells (PD331/C), as demonstrated by lower TCF/LEF reporter activation after GSK3β inhibition using LiCl or CT99021 (Fig. 4I). Taken together, our results suggest that FANCC is required for efficient β-catenin nuclear entry and subsequent activity. Consequently, FANCC may affect the transcriptional regulation of β-catenin target genes.
FA Proteins Act as Transcriptional Repressors of DKK1.
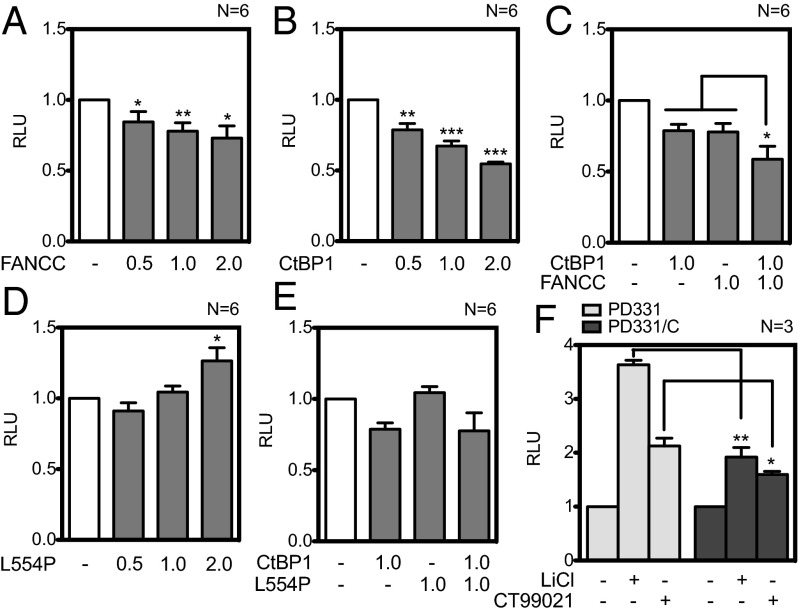
Based on our previously reported data showing abnormal expression of Wnt/β-catenin target genes, specifically DKK1, in FA-deficient cells (7), we investigated the role of FANCC in the transcriptional regulation of DKK1. To do so, we cloned the human DKK1 promoter into the pGL3 luciferase reporter vector. First, we transfected the DKK1 reporter construct in cells expressing increasing amounts of FANCC. Our results show that FANCC was able to significantly repress DKK1 reporter activity in a dose-dependent manner (Fig. 5A), but to a lesser extant than CtBP1, a known repressor (Fig. 5B). These results suggest that FANCC transcriptional repression activity requires a cofactor. Indeed, cotransfection of FANCC with CtBP1 led to further decrease in DKK1 transcriptional activity (Fig. 5C).
Fig. 5.
FANCC and CtBP1 act as transcriptional repressors of DKK1. (A–E) Luciferase assays performed in HeLa cells transfected with the DKK1 promoter reporter construct along with equimolar amounts of β-catenin and TCF4 expression vectors and with control empty vectors or various concentrations of FANCC (A), CtBP1 (B), FANCC with CtBP1 (C), FANCCL554P (D), or FANCCL554P with CtBP1 (E) as indicated. (F) Luciferase assays performed in patient-derived FANCC-deficient (PD331) and FANCC-corrected (PD331/C) cells transfected with the DKK1 promoter reporter construct and treated with CT99021 or LiCl. The number of experiments performed in duplicate is indicated in each graph. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
We next evaluated the repression capacity of FANCC harboring the L554P disease-causing mutation (FANCCL554P; Fig. 5D). Increasing amounts of FANCCL554P lead to a dose-dependent activation of the DKK1 promoter, suggesting that the mutated form of FANCC lost its capacity to repress DKK1. Indeed, FANCCL554P had no effect on CtBP1-mediated transcriptional repression of the DKK1 promoter (Fig. 5E). These results imply that CtBP1 and FANCC act together as negative regulators of DKK1 expression, and that disease-causing mutations in FANCC negatively affect this function. Indeed, this idea is supported by results obtained in patient-derived FANCC-deficient cells (PD331), whereas stronger DKK1 promoter activation is found compared with that in FANCC-corrected cells (PD331/C) (Fig. 5F). These results suggest that FANCC with the corepressor CtBP1 negatively regulates DKK1 expression.
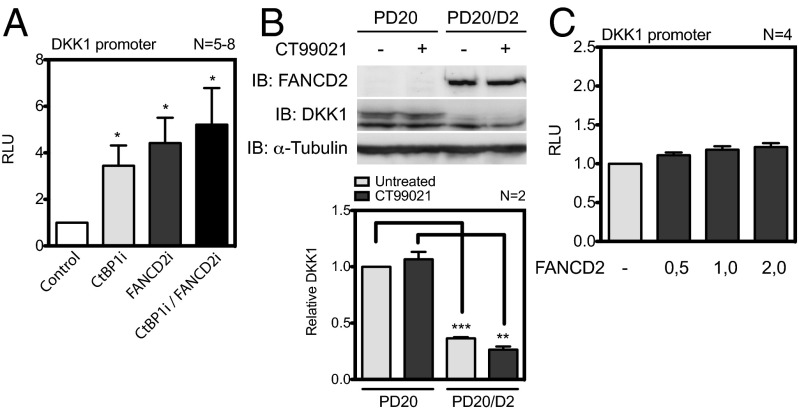
To determine whether efficient DKK1 repression requires a functional FA pathway, we evaluated DKK1 transcriptional activity in FA-deficient cells. Our results show that the silencing of FANCD2 led to a fivefold induction of DKK1 reporter activity similar to that found in FANCC mutant cells and CtBP1-depleted cells (Fig. 6A). Silencing of FANCD2 and CtBP1 led to a further increase in DKK1 reporter activation. These results are consistent with our previous findings (7) showing a threefold to fourfold increase in DKK1 mRNA and protein expression in FANCD2-depleted cells. Western blot analyses performed in patient-derived FANCD2-mutant cells (PD20) confirmed the results obtained in FANCD2i cells showing elevated levels of DKK1, whereas complementation with the FANCD2 gene (PD20/D2) reduced DKK1 protein levels to normal (Fig. 6B). These results suggest that a functional FANCD2 protein is required for DKK1 repression; however, increasing amounts of the FANCD2 protein did not significantly impact the DKK1 reporter, suggesting an indirect effect (Fig. 6C). Taken together, these results suggest that the FA pathway acts as a transcriptional repressor of DKK1.
Fig. 6.
FANCD2-deficient cells have increased DKK1 expression. (A) Luciferase assays performed in FANCD2i, CtBP1i, FANCD2i/CtBP1i, or control cells (control) transfected with the DKK1 reporter and assayed for luciferase activity at 24 h after transfection. (B) Western blot analysis of WCEs from PD20 and PD220/D2 cells treated with CT99021 with the indicated antibodies (Upper). Mean relative expression of the DKK1/α-tubulin ratio ± SEM relative to untreated cells from two independent blots (Lower). **P ≤ 0.01; ***P ≤ 0.001. (C) Luciferase assays performed in HeLa cells transfected with the DKK1 reporter along with β-catenin, TCF4 expression vectors, and increasing concentrations of FANCD2, as indicated.
Discussion
We recently reported an interaction between the FANCC protein and the transcriptional corepressor CtBP1 (7). Our previous study highlighted the implications of FA proteins along with CtBP1 in the transcriptional regulation of Wnt pathway-responsive genes including DKK1, a Wnt antagonist. Because DKK1 is a target of TCF/β-catenin–mediated transcription as well as a Wnt antagonist acting as a negative feedback loop (10, 24), we investigated the functional implication of FA proteins in β-catenin signaling and DKK1 transcriptional regulation. In the present study, we have demonstrated that FANCC forms a complex with β-catenin and CtBP1. We also have shown that FANCC localizes to the nucleus with both β-catenin and CtBP1 after activation of β-catenin either by its overexpression or by inhibition of GSK3β. We also provide evidence that FANCC is required for the efficient nuclear translocation of β-catenin after GSK3β inhibition. These results imply that efficient nuclear accumulation of β-catenin would be prevented in cells with a defective FA pathway.
Indeed, we observed that in FANCA- and FANCD2-depleted cells, as well as in patient-derived FA mutant cells, the majority of β-catenin remains restricted to the cytoplasm after activation of the Wnt pathway. These results imply that the lack of a functional FANCC protein or the absence of a functional FA pathway would impede the transcriptional activity of β-catenin. As expected, transcriptional activation of the β-catenin/TCF reporter was reduced in patient-derived FA mutant cells compared with their corrected counterparts. These data are consistent with our previous results showing reduced β-catenin/TCF reporter activation in FA-depleted cells (7).
In addition, we found that overexpression of FA core complex components triggered transcriptional activation of the β-catenin/TCF reporter in dose-dependent manner, whereas overexpression of only FANCC had no effect on β-catenin/TCF reporter activation. Furthermore, overexpression of FANCD2 promoted transcription of the β-catenin/TCF reporter similar to that of FA core complex components, suggesting that the FA core complex-mediated activation of the β-catenin/TCF reporter occurs through the FA pathway downstream component FANCD2. In addition, we observed that GSK3β inhibition triggered activation of the FA pathway as measured by formation of the long form of FANCD2. Recent studies support a role of the FA core complex via FANCD2 in transcription, both activation and repression (25–27). Taken together, those studies and our results imply that FA core complex activity may modulate β-catenin function. Indeed, this is supported by a study by Dao et al. (23), which showed that β-catenin activity is facilitated by FA core complex ubiquitin ligase activity. Our findings support a role of the FA pathway in β-catenin nuclear accumulation and subsequent activity.
Our findings suggest that a defective FA pathway leading to diminished nuclear β-catenin and, consequently, diminished β-catenin/TCF activity would negatively affect β-catenin target genes. Indeed, our previous microarray data showed that FA-deficient cells demonstrate down-regulation of Wnt/β-catenin target genes involved in signal activation. However, we also observed that other Wnt signal modulators, such as DKK1, were up-regulated in these FA-deficient cells (7), suggesting a dual function of FA proteins. Our present data provide evidence of transcriptional regulation of DKK1 by FANCC, as demonstrated by dose-dependent diminished DKK1 promoter activation after increasing amounts of FANCC. In addition, overexpression of FANCC and CtBP1 led to further DKK1 transcriptional repression.
Consistent with the role of FANCC in DKK1 repression, the FANCC protein harboring the disease-causing mutation L554P failed to repress transcription of the DKK1 promoter. Moreover, patient-derived FANCC mutant cells (PD331) showed increased DKK1 transcriptional activation. These effects would result in elevated levels of DKK1. Indeed, we previously showed that FancC-deficient mice have increased Dkk1 serum levels (7). Taken together, our data suggest that FANCC with CtBP1 acts as a corepressor of DKK1.
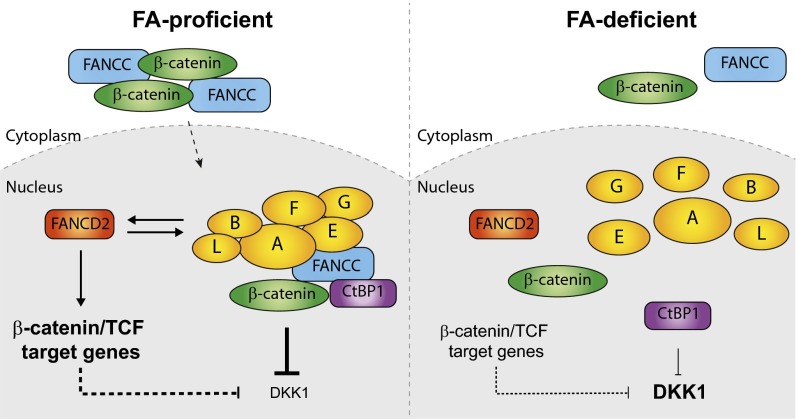
Our findings support a model (Fig. 7) in which the FA pathway acts in transcriptional regulation of the DKK1 gene. Here we show that FANCC accumulates in the nucleus in response to β-catenin activation. Once in the nucleus, FANCC and its binding partner CtBP1 repress transcription of DKK1. Concomitantly, via FANCD2, the FA core complex enhances the transcriptional activation of β-catenin/TCF target genes and subsequently represses DKK1. Consequently, the absence of a functional FA pathway would prevent FANCC from entering the nucleus and repressing the DKK1 gene, leading to overproduction of DKK1. In addition, lack of a functional FA core complex would lead to reduced activation of β-catenin/TCF target genes and DKK1 repression. Overall, our data provide evidence of a dual mechanism through which the FA pathway acts in transcriptional regulation of the DKK1 gene.
Fig. 7.
Proposed model of a dual mechanism by which the FA pathway regulates expression of DKK1. (Left) In the presence of an intact FA pathway (FA-proficient), FANCC and β-catenin efficiently accumulate and localize into the nucleus after GSK3β inhibition. In the nucleus, FANCC forms a complex with CtBP1 and β-catenin and represses DKK1. The FA core complex via FANCD2 enhances expression of other β-catenin/TCF target genes. FANCD2 influences the stability or expression of FA core complex proteins and, subsequently, DKK1 expression. (Right) In the absence of a functional FA pathway (FA-deficient), FANCC and β-catenin do not efficiently accumulate into the nucleus. As a result, FANCC fails to efficiently repress DKK1. Lack of FA core complex activity results in reduced expression of β-catenin/TCF targets and increased DKK1 expression.
Our results are relevant to the FA disease, because overproduction of Dkk1 from bone marrow niche cells has been associated with altered HSC function, leading to impaired self-renewal capacity (17). Impaired self-renewal capacity is a hallmark of FA-deficient HSCs (20–22). In addition, overproduction of Dkk1 induces cell cycling of primitive hematopoietic cells that generally are maintained in a quiescent state (17, 28). FA-deficient primitive hematopoietic cells were shown to have an accelerated cell cycle (29). Taken together, these findings support our model and provide a clue to explaining the failure of bone marrow in patients with FA. Further investigation is needed to determine whether DKK1 serves as a potential therapeutic target to prevent exhaustion of primitive bone marrow cells.
Materials and Methods
Cell Lines and Culture Conditions.
HEK293T (American Type Culture Collection and Cedarlane Laboratories), HeLa (American Type Culture Collection), and PD432, PD720 (FA-A), PD331 (FA-C), PD20 (FA-D2), and PD20-corrected (PD20/D2) (gifts from Dr. Markus Grompe, Oregon Health Science University, Portland, OR and the Fanconi Anemia Research Fund) fibroblast cell lines were cultured in DMEM supplemented with 10% FBS and grown at 37 °C in a humidified atmosphere containing 5% CO2. The knockdown of CtBP1 (CtBP1i) and FA genes (FANCAi and FANCD2i) in HeLa cells was performed using different pLKO.1 plasmids carrying shRNAs targeting FANCA (TRCN0000118982), FANCD2 (TRCN0000082840), or CtBP1 (TRCN0000013738), as described previously (7). A lentivirus expression system was used to stably complement PD331 with WT FANCC cDNA (PD331/C). Functional correction of PD720 cells was done by transfection of the FANCA-coding plasmid (PD720/A). Stealth small inhibitory RNAs (siRNAs) for use against CtBP1 and the negative control were purchased from Invitrogen. Where indicated, cells were exposed to GSK3β inhibitors LiCl (Sigma-Aldrich), BIO/GSK3 inhibitor IX (Calbiochem), CT99021 (Cayman Chemical), or mitomycin C (Sigma-Aldrich). Cells were treated with LiCl at 100 mM for 3 h (short exposure) or 50 mM for 16 h (long exposure). Cells were treated with the BIO/GSK3β inhibitor IX at 5 μM for 16 h. CT99021 was used at 10 μM for 3 h (short exposure) or at 1 μM for 16 h (long exposure), and mitomycin C was used at 100 nM for 16 h.
DNA Constructs and Antibodies.
All FA gene constructs used in this study have been described previously (7, 30). The pCEP4-FANCCL554P plasmid was generously provided by Dr. Maureen Hoatlin, Oregon Health & Science University, Portland, OR. The FANCD2 expression plasmid (pIRESneo-FANCD2) was generously provided by Markus Grompe, Oregon Health & Science University. The pRc/CMV-T7-CtBP1 plasmid was a gift from Dr. G. Chinnadurai, Saint Louis University Health Sciences Center, St. Louis, MO. The pCMV6-XL5-β-catenin plasmid was obtained from OriGene Technologies. The TCF4 expression construct was obtained from Upstate Cell Signaling Solutions. The pGL3-basic was obtained from Promega. The following antibodies were used: anti-FANCA (C-20; Santa Cruz Biotechnology or Novus Biologicals), anti-FANCC (31) (8F3, a gift from Dr. M. Hoatlin, Oregon Health & Science University, Abcam, or Novus Biologicals), anti-FANCD2 (Novus Biologicals), anti-CtBP1 (Millipore or BD Biosciences), anti–β-catenin (R&D Systems or Santa Cruz Biotechnology), anti-DKK1 (R&D Systems), anti-hemagglutinin (HA) (12CA5; Roche Diagnostics), anti-cMyc (9E10; Santa Cruz Biotechnologies), anti–α-tubulin (Sigma-Aldrich), anti–TATA-binding protein (TBP; Abcam), anti-goat (Calbiochem), anti-mouse and anti-rabbit (Santa Cruz Biotechnologies), and donkey anti-rabbit Alexa Fluor 488, anti-mouse Alexa Fluor 555, and anti-goat Alexa Fluor 680 (Invitrogen).
Cellular Fractionation and Immunoprecipitation.
Whole-cell extracts (WCEs) from HEK293T or HeLa cells were subjected to IP, immunoblot analysis, or cell fractionation studies as described previously (30). For IP, equal amounts of protein were incubated overnight at 4 °C with 2 µg of antibodies, followed by incubation with protein G or A-agarose beads (Calbiochem) or protein-G magnetic beads (Invitrogen). Immunoprecipitates were resolved by SDS/PAGE and subjected to Western blot analysis with specific antibodies, as indicated in each figure. For cell fractionation studies, protein extracts were subjected to cellular separation and preparation using NE-PER nuclear and cytoplasmic extraction reagents (ThermoScientific) according to the manufacturer’s instructions.
Immunofluorescence.
For detecting the cellular localization of FANCC, β-catenin, and CtBP1, HeLa, PD432, PD720, PD720/A, PD331, and PD331/C cells were either fixed in methanol-acetone (3:7 vol/vol) or in 4% paraformaldehyde and permeabilized with 0.1% saponin or 0.3% Triton X-100 before standard immunofluorescent staining. The slides were mounted with DAPI-Fluoromount-G (Southern Biotech) for fluorescence microscopy. Cell nuclei were labeled with TOPRO-3 (Invitrogen) or DAPI (Sigma-Aldrich) for confocal microscopy. Cells were visualized with a Nikon E800 fluorescent microscope equipped with a C1 confocal system (Nikon Canada) or were observed under a Zeiss Axio Imager M2 microscope equipped with an AxioCam MRm digital camera and AxioVision 4.8 software (Zeiss).
Transcriptional Reporter Assays.
The DKK1 promoter spanning the −1037 to +163 region of the gene was amplified by PCR using the forward sequence primer 5-CTCCCTAGAAAGGGTATTG-3 and the reverse primer 5-AGATAGGACCCTTTCAAGG-3, and then cloned into the pGL3-basic luciferase reporter vector (pGL3-DKK1). For the DKK1 promoter reporter or TCF/LEF reporter assays, cells were transfected with the plasmids pGL3-DKK1 or M50 Super 8X TOPFlash (Addgene; plasmid 12456) along with the Renilla luciferase control plasmid (Promega). The total amount of plasmid DNA was equalized between the transfections using empty vectors.
For β-catenin–mediated activation of DKK1 promoter and for the TCF/LEF reporter assays, cells were treated at 16 h after transfection of the luciferase reporter vectors with LiCl (50 mM) or CT99021 (10 μM). Cell extracts were prepared at 24–48 h after transfection and assayed for luciferase activity with the Promega Dual Luciferase Reporter Assay System according to the manufacturer’s instructions, using an automated plate reader (Tecan Infinite 200). Luciferase activity was normalized to Renilla luciferase and expressed as the mean fold change ± SEM relative to control cells.
Statistical Analyses.
Data are expressed as mean ± SEM. Statistical analyses were performed using GraphPad Prism version 5.0b, and paired and unpaired two-tailed Student t tests were used to compare the means. A P value < 0.05 was considered significant.
Acknowledgments
We thank Dr. M. Hoatlin for providing the anti-FANCC 8F3 monoclonal antibodies and the pCEP4-HA-FANCCL554P and Dr. M. Grompe for FA cells and the FANCD2 expression plasmid. We thank Annie Gagnon for her work related to siRNA against CtBP1 and Marie-Chantal Delisle for her technical expertise. This work was supported in part by grants from the Canadian Institutes of Health Research in partnership with the Canadian Blood Services Blood Utilisation and Conservation Initiative grant (M.C. and G.L.), the Canadian Leukemia and Lymphoma Society, and scholarships from Canadian Institutes of Health Research in partnership with the Canadian Fanconi Anemia Research Fund (C.C.H.) and the Foundation of Stars (C.C.H., C.S.T., and A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.H. is a guest editor invited by the Editorial Board.
References
- 1.Bagby GC, Lipton JM, Sloand EM, Schiffer CA. Marrow failure. Hematology (Am Soc Hematol Educ Program) 2004;2004(1):318–336. doi: 10.1182/asheducation-2004.1.318. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald M, Carreau M. Genetic basis of Fanconi anemia. In: Schrezenmeier H, Bacigalupo A, editors. Aplastic Anemia. Cambridge, UK: Cambridge Univ Press; 1999. p. 403. [Google Scholar]
- 3.Bogliolo M, et al. Mutations in ERCC4, encoding the DNA-repair endonuclease XPF, cause Fanconi anemia. Am J Hum Genet. 2013;92(5):800–806. doi: 10.1016/j.ajhg.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashiyama K, et al. Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia. Am J Hum Genet. 2013;92(5):807–819. doi: 10.1016/j.ajhg.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kee Y, D’Andrea AD. Molecular pathogenesis and clinical management of Fanconi anemia. J Clin Invest. 2012;122(11):3799–3806. doi: 10.1172/JCI58321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaddar T, Carreau M. Fanconi anemia proteins and their interacting partners: A molecular puzzle. Anemia. 2012;2012:425814. doi: 10.1155/2012/425814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huard CC, et al. Fanconi anemia proteins interact with CtBP1 and modulate the expression of the Wnt antagonist Dickkopf-1. Blood. 2013;121(10):1729–1739. doi: 10.1182/blood-2012-02-408997. [DOI] [PubMed] [Google Scholar]
- 8.Chinnadurai G. Transcriptional regulation by C-terminal binding proteins. Int J Biochem Cell Biol. 2007;39(9):1593–1607. doi: 10.1016/j.biocel.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, et al. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature. 2003;422(6933):735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 10.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 11.Glinka A, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay M, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1(3):423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: Dose dependence and compensatory interactions with Lrp6. Development. 2004;131(11):2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- 14.Adamska M, MacDonald BT, Meisler MH. Doubleridge, a mouse mutant with defective compaction of the apical ectodermal ridge and normal dorsal-ventral patterning of the limb. Dev Biol. 2003;255(2):350–362. doi: 10.1016/s0012-1606(02)00114-8. [DOI] [PubMed] [Google Scholar]
- 15.Pinzone JJ, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113(3):517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alter BP. Cancer in Fanconi anemia, 1927-2001. Cancer. 2003;97(2):425–440. doi: 10.1002/cncr.11046. [DOI] [PubMed] [Google Scholar]
- 17.Fleming HE, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alter BP, Joenje H, Oostra AB, Pals G. Fanconi anemia: Adult head and neck cancer and hematopoietic mosaicism. Arch Otolaryngol Head Neck Surg. 2005;131(7):635–639. doi: 10.1001/archotol.131.7.635. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg PS, Socié G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105(1):67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 20.Carreau M, et al. Hematopoietic compartment of Fanconi anemia group C null mice contains fewer lineage-negative CD34+ primitive hematopoietic cells and shows reduced reconstruction ability. Exp Hematol. 1999;27(11):1667–1674. doi: 10.1016/s0301-472x(99)00102-2. [DOI] [PubMed] [Google Scholar]
- 21.Haneline LS, et al. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood. 1999;94(1):1–8. [PubMed] [Google Scholar]
- 22.Habi O, Delisle MC, Messier N, Carreau M. Lack of self-renewal capacity in Fancc−/− stem cells after ex vivo expansion. Stem Cells. 2005;23(8):1135–1141. doi: 10.1634/stemcells.2004-0356. [DOI] [PubMed] [Google Scholar]
- 23.Dao KH, et al. FANCL ubiquitinates β-catenin and enhances its nuclear function. Blood. 2012;120(2):323–334. doi: 10.1182/blood-2011-11-388355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semënov MV, Zhang X, He X. DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem. 2008;283(31):21427–21432. doi: 10.1074/jbc.M800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du W, et al. The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters. Blood. 2012;119(18):4142–4151. doi: 10.1182/blood-2011-09-381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park E, et al. FANCD2 activates transcription of TAp63 and suppresses tumorigenesis. Mol Cell. 2013;50(6):908–918. doi: 10.1016/j.molcel.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita N, et al. Direct inhibition of TNF-α promoter activity by Fanconi anemia protein FANCD2. PLoS ONE. 2011;6(8):e23324. doi: 10.1371/journal.pone.0023324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278(30):28067–28078. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- 29.Li X, et al. Fanconi anemia type C-deficient hematopoietic stem/progenitor cells exhibit aberrant cell cycle control. Blood. 2003;102(6):2081–2084. doi: 10.1182/blood-2003-02-0536. [DOI] [PubMed] [Google Scholar]
- 30.Tremblay CS, et al. HES1 is a novel interactor of the Fanconi anemia core complex. Blood. 2008;112(5):2062–2070. doi: 10.1182/blood-2008-04-152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodeur I, et al. Regulation of the Fanconi anemia group C protein through proteolytic modification. J Biol Chem. 2004;279(6):4713–4720. doi: 10.1074/jbc.M301291200. [DOI] [PubMed] [Google Scholar]