Fig. 3.
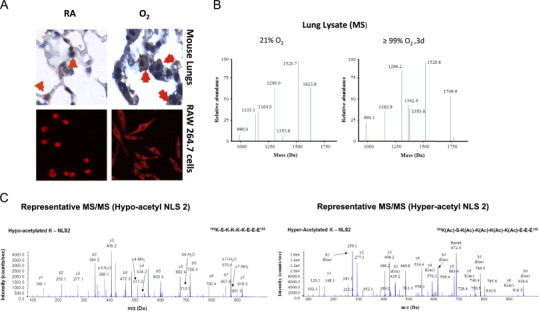
Hyperoxia induces hyperacetylation and translocation of nuclear HMGB1 to the cytoplasm. C57BL/6 mice were exposed to either 4 days of ≥99% O2 or remained at RA. (A) Images of lung tissue sections (original magnification, X1000) stained with anti-HMGB1 IgGs and DAB (3, 3'-diaminobenzidine) peroxidase substrate (brown color). The red signals showed immunofluoresence staining with anti-HMGB1 IgGs and Cy3 in RAW 264.7 macrophages that were either exposed to 95% O2 for 48 h or remained at RA (original magnification, X600). (B) Representative spectra of the liquid chromatography mass spectrometric (LC–MS) characterization of peptides produced from HMGB1 derived from mice lung tissue homogenate (Lung Lysate) enzymatically cleaved with endopeptidase GluC. The presence of the peptides with molecular weights 1624 and 1133 Da indicate the hypo-acetylation of lysine residues within NLS 1 and 2, respectively. The presence of the peptides with molecular weights 1750 and 1342 Da indicate the hyper-acetylation of lysine residues within NLS 1 and 2 respectively. (C) Representative spectra of the liquid chromatography tandem mass spectrometric (LC–MS/MS) characterization of a peptide (amino acids 180–188) covering the lysine (K) residues within NLS 2 of HMGB1 to confirm the presence or absence of acetyl modifications on specific K residues. Acetyl modifications are represented as (ac) on specific lysine residues (K181, K182, K183 and K184) when required and b and y ions are highlighted were appropriate.
