Fig. 4.
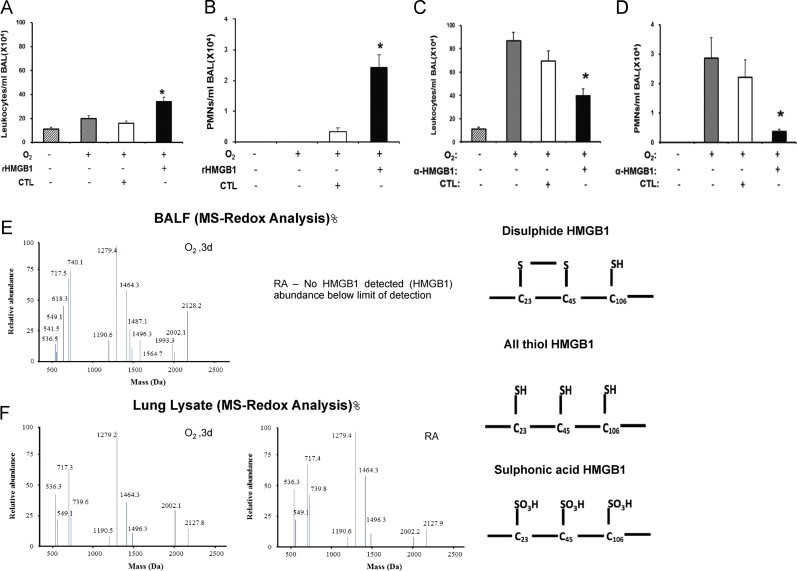
Hyperoxia increases oxidative states of airway HMGB1 that induces leukocyte infiltration into the lungs. C57BL/6 mice were given 10 μg rHMGB1- or gluthatione-s-transferase (GST-tag) as control (CTL) via intratracheal instillation 24 h post-hyperoxic (≥99% O2) exposure. Mice were then exposed to hyperoxia for an additional 24 h. Total numbers of leukocytes and PMNs infiltrated into the airways were analyzed as markers of inflammatory ALI. Data represent means±SE from two independent experiments, n=9 mice per group. ⁎, Statistically significant vs. the values of the control group which received GST-tag, P<0.05 (A and B). Mice were treated intraperitonealy with either 360 μg/mouse anti-HMGB1 IgGs (α-HMGB1) or control IgGs (CTL) 2 h prior to hyperoxic exposure. Mice were then exposed to ≥99% O2 and received either α-HMGB1 IgGs or control IgGs intraperitonealy every 12 h for 4 days while still being exposed to ≥99% O2. Total numbers of leukocytes and PMNs infiltrated into the airways were analyzed. Data represent means±SE from two independent experiments, n=9 mice per group. ⁎, Statistically significant vs. mice treated with control IgGs, P<0.05 (C and D). BALF and lung tissue from mice exposed to ≥99% O2 for 3 days or that remained at RA, were analyzed for the presence of three redox forms of HMGB1: disulfide, all thiol and sulfonic acid using LC–MS (E and F).
