Fig. 3.
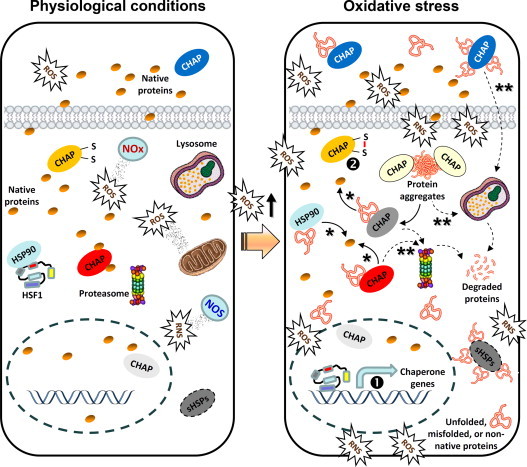
Free radicals (e.g. ROS or RNS) are derived from exogenous (e.g. environmental) as well as intracellular (e.g. mitochondria, NOS, NOx) sources and at physiological concentrations play a significant role as regulatory mediators in signalling pathways. Under these conditions of minimal proteotoxic stress, chaperones (CHAP) are expressed at basal levels and HSF1 is at an inert state by binding to chaperones (e.g. HSP90). A sustained increase in ROS beyond a physiological threshold (redox imbalance) results in increased oxidative and proteotoxic stress. Consequently, HSF1 liberates from HSP90 (which now preferentially binds stressed proteins) and translocates to the nucleus to enhance the expression levels of chaperone genes (transcriptional regulation; ❶). Also, chaperones can be activated at a post-translational level by cycling between a low- and high-affinity substrate binding state, depending on the redox state of their cysteines (❷). Chaperone bound extra- or intra-cellular stressed polypeptides can be either refolded into their native form (⁎) or targeted to proteases for degradation (⁎⁎); similarly, accumulating protein aggregates can be disaggregated to unfolded intermediates and either refolded or targeted for degradation.
