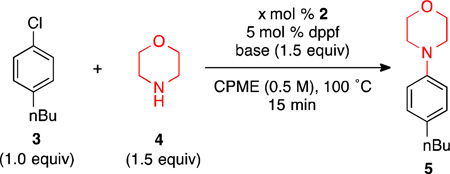
Table 1.
Optimization of Reaction Conditionsa
 | |||||
|---|---|---|---|---|---|
| entry | mol % 2 | additive | base | conversionb | yieldb |
| 1 | 5 | None | NaOtBu | 21% | 10% |
| 2 | 5 | None | KOtBu | 23% | 9% |
| 3 | 5 | None | LiOtBu | 54% | 49% |
| 4 | 5 | MeCN | LiOtBu | 90% | 86% |
| 5c | 5 | MeCN | LiOtBu | 100% | 91% |
| 6 | 5 | MeCN | NaOtBu | 14% | 5% |
| 7 | 5 | MeCN | KOtBu | 4% | <4% |
| 8d | 5 | MeCN | LiOtBu | 79% | 68% |
| 9 | 2.5 | MeCN | LiOtBu | 58% | 50% |
| 10e | 2.5 | MeCN | LiOtBu | 68% | 60% |
Reaction conditions: 3 (0.25 mmol), 4 (0.375 mmol), base (0.375 mmol) 2 (2.5 – 5 mol %), dppf (2.5 – 5 mol %), additive (0.25 mmol), CPME (0.5 mL), 100 °C, 15 min.
Determined by GC using dodecane as the internal standard.
Reaction time was 45 min. Isolated yield was 85%, 1 mmol scale, average of two runs.
No additional dppf was added.
Reaction time was 1 h.
CPME = cyclopentyl methyl ether.
