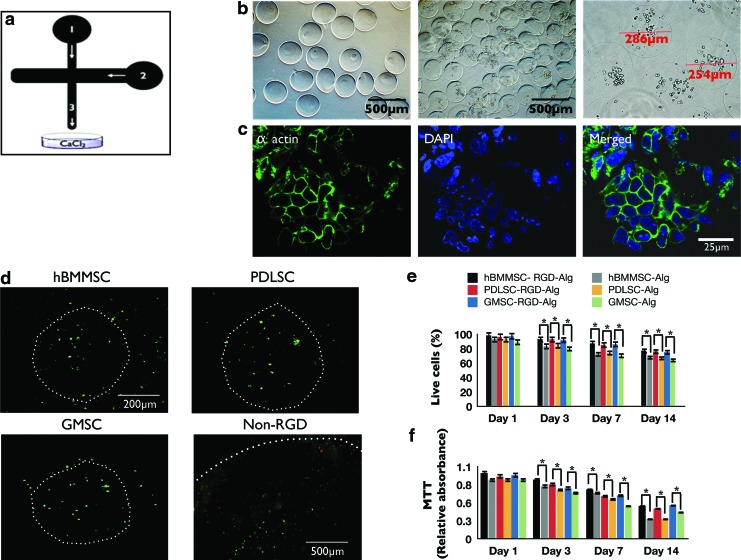
FIG. 2.
(a) Schematic representation of microfluidic device. Channel 1: alginate injection channel; Channel 2: soybean oil injection channel. Alginate droplets were sheared off by the soybean oil and flowed out of the device through Channel 3. (b) Microspheres produced by the microfluidic device. The diameter of the microspheres was between 196 and 581 μm (scale bar=500 μm). (c) Cytoskeleton organization of MSCs encapsulated in alginate microspheres stained with phalloidin Alexa Fluor 568 for F-actin (green) and 4′,6-diamidino-2-phenylindole (DAPI, blue) for nucleus. (d) Live/dead staining of the stem cell microspheres after 1 week of culturing (scale bar=200 μm). White dots show the peripheries of each microcapsule. Note the larger diameter of the non-RGD-containing alginate microsphere (with PDLSCs) with average diameter of 1 mm, fabricated via traditional methods.18,19 Larger microspheres show more dead positive cells after 1 week than RGD-coupled microspheres fabricated using microfluidics. (e) Viability of the encapsulated PDLSCs, GMSCs, and hBMMSCs: live/dead staining, percentage of live cells in either RGD-coupled alginate microspheres or in alginate microspheres without RGD. (f ) 3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay of metabolic activity of cells. No significant difference was observed between the stem cell groups at each time interval. *p<0.05. Color images available online at www.liebertpub.com/tea