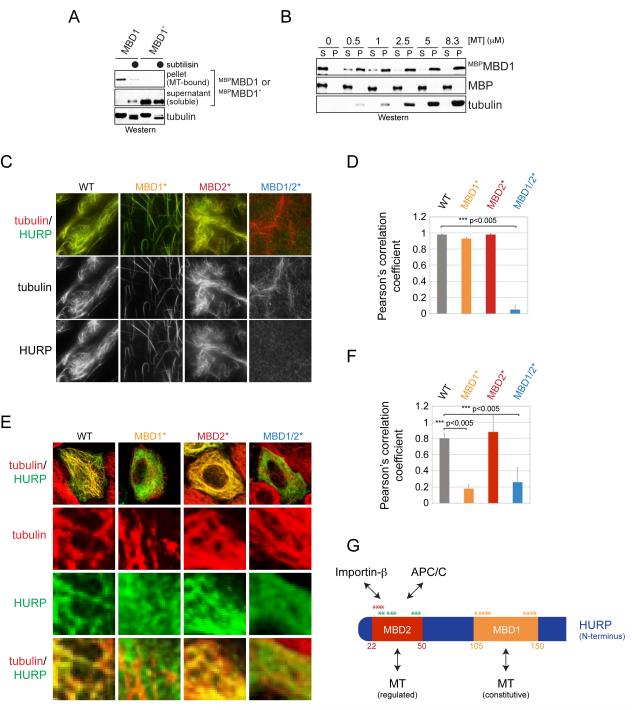
Figure 3. HURP contains two microtubule-binding motifs.
A. Taxol-stabilized microtubules were incubated with MBPMBD1 or mutant MBPMBD1* in which positively charged residues were exchanged to alanine. As indicated, microtubules were treated with subtilisin. Binding reactions were centrifuged through a sucrose cushion, and microtubule-bound and soluble fractions were analyzed by Western. B. The MBD1 binds microtubules with similar affinity as full-length HURP. MBPMBD1 was incubated with taxol-stabilized microtubules and subjected to sucrose gradient centrifugation. C. The MBD1 and MBD2 both mediate microtubule-binding of HURP. Recombinant full-length HURP or mutants in its MBD1 or MBD2 were labeled with Oregon Green and incubated with rhodamine-labeled microtubules. Binding was analyzed by fluorescence microscopy. D. Quantification of HURP- and microtubule co-localization in vitro. Green (HURP) and red (tubulin) signals were correlated using ImageJ and JACoP, resulting in a Pearson’s correlation coefficient. E. MBD1 mediates microtubule-binding of HURP in interphase. HeLa cells were transfected with FLAGHURP or mutants in its MBDs and analyzed by immunofluorescence microscopy (green: HURP; red: β-tubulin). F. Pearson’s correlation coefficient to quantify the co-localization between HURP-mutants and microtubules in interphase cells. G. Overview of binding motifs in the N-terminal domain of HURP. The MBD1 provides a constitutive binding site for microtubules; MBD2 overlaps with APC/C-degrons and the importin-β-binding site (Song and Rape, 2010). The asterisks mark residues mutated to alanine in order to ablate the functions of the MBD1 (orange), MBD2 (red), or APC/C-degrons (green). See also Figure S2.