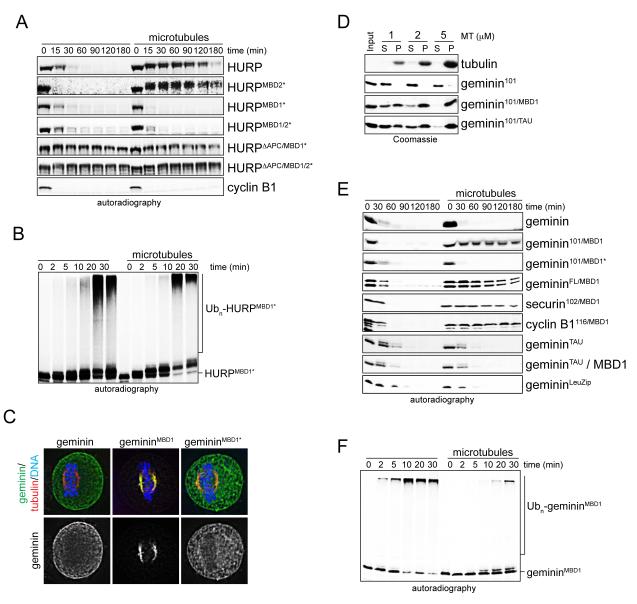
Figure 4. The MBD1 is required and sufficient for the regulation of HURP-stability by microtubules.
A. MBD1-mutation allows degradation of HURP in the presence of microtubules. Mitotic extracts with APC/CCdc20 and RanQ69L were supplemented with 35S-labeled HURP, HURP-mutants (MBD1*, MBD2*, ΔAPC), or cyclin B1, and degradation was monitored by autoradiography. As indicated, microtubules were stabilized by taxol. B. The MBD1 is required for the inhibition of HURP-ubiquitylation by microtubules. 35S-labeled HURPMBD1* was incubated with buffer or microtubules, before being subjected to ubiquitylation by APC/CCdc20. Reactions were followed by autoradiography. C. The MBD1 targets a soluble APC/C-substrate to the spindle. HeLa cells were transfected with geminin, gemininMBD1 (residues 1-101 of geminin fused to the MBD1 of HURP); or gemininMBD1* (charged residues in the MBD1 mutated to alanine), and localization was determined by immunofluorescence microscopy (green: geminin; red: tubulin; blue: DNA/DAPI). The bottom panel shows the localization of the geminin proteins alone. D. Fusions of HURP’s MBD1 or the N-terminal microtubule-binding domain of tau induce microtubule-binding of soluble APC/C-substrates with similar efficiency. Binding of recombinant proteins to microtubules was analyzed by sucrose gradient centrifugation (S: soluble fraction; P: microtubule-bound fraction). E. The MBD1 imposes microtubule-dependent regulation of degradation. 35S-labeled substrates were added to extracts with APC/CCdc20 and RanQ69L (MBD1: HURP’s MBD1; TAU: N-terminal microtubule-binding domain of tau; LeuZip: leucine zippers of GCN4 transcription factor). As indicated, microtubules were stabilized by taxol or the MBPMBD1 was added to induce microtubule bundling. Reactions were monitored by autoradiography. F. Microtubules inhibit the ubiquitylation of gemininMBD1. 35S-labeled gemininMBD1 was incubated with buffer or microtubules and subjected to ubiquitylation by APC/CCdc20. Reactions were monitored by autoradiography. See also Figure S3.