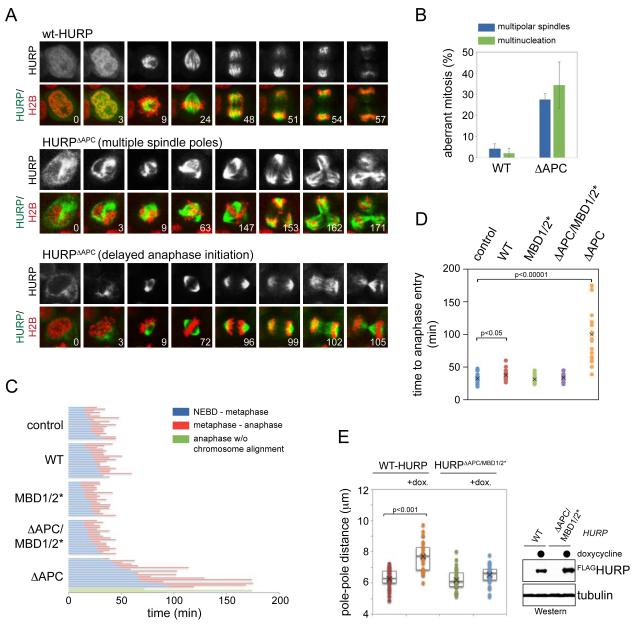
Figure 6. Stabilization of HURP interferes with spindle structure and function.
A. Expression of stabilized HURP in mitosis leads to persistent microtubule-binding and mitotic defects. HeLa cells transduced with lentiviruses expressing mCherryhistone H2B, and GFP-tagged HURP or HURPΔAPC, were filmed through mitosis. Upper panel: HURP; lower panel: GFP-tagged HURP (green), mCherryhistone H2B (red), and the time post nuclear envelope breakdown. B. Stabilization of HURP results in multiple spindle poles and multinucleation. Cells expressing GFP-HURP or GFP-HURPΔAPC were filmed through mitosis and analyzed for >2 spindle poles or multinucleation. Results were from three independent experiments, including at least 50 dividing cells per condition and per experiment. C. Expression of HURPΔAPC delays the assembly of a metaphase plate with completely aligned chromosomes. HeLa cells expressing GFP-tagged HURP or mutants were filmed through mitosis. The time from nuclear envelope breakdown to assembly of a metaphase plate (blue) and from metaphase to anaphase initiation (red) are shown. Expression of HURPΔAPC frequently caused anaphase initiation after a profound delay, even though a metaphase plate had never been established (green). D. Quantification of the time required for anaphase entry in the presence of HURP proteins. E. Increased levels of active HURP cause spindle defects. Stable 293T cell lines expressing HURP or HURPΔAPC/MBD1/2* under an inducible promoter were treated with doxycycline, and spindle structures were analyzed by microscopy against α- and γ-tubulin. Left panel: pole-pole distance in the presence or absence of HURP; right panel: inducible expression of HURP-variants. See also Figure S5.