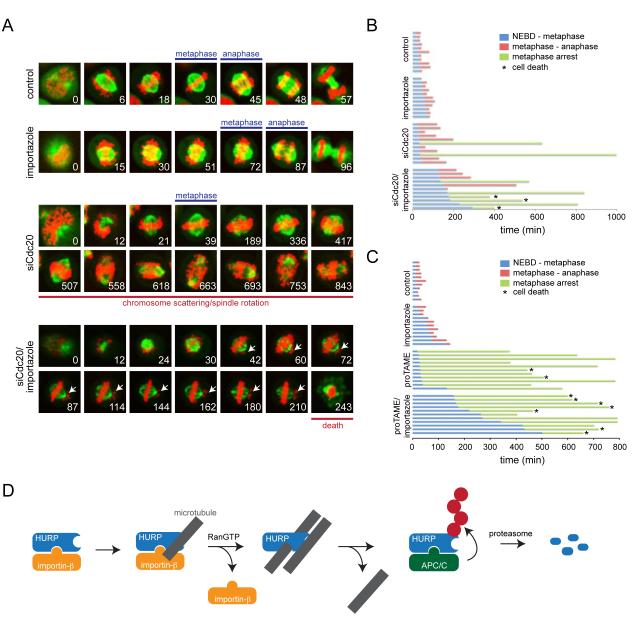
Figure 7. The APC/C and importin-β regulate spindle function.
A. Simultaneous inhibition of the APC/C and importin-β results in cooperative defects in spindle structure and function. HeLa cells stably expressing GFPtubulin and mCherryhistone H2B were treated with siRNAs against Cdc20, importazole, or siRNAs against Cdc20 and importazole at the same time. Progression of cells through mitosis was monitored by video microscopy (tubulin: green; histone: red; arrows: misaligned chromosomes). B. Quantification of the mitotic timing for HeLa cells expressing mCherryhistone H2B and GFPtubulin, after being treated with siRNAs against Cdc20 or importazole, as shown on the left. Blue: time from nuclear envelope breakdown to completion of chromosome alignment; red: time from metaphase to anaphase initiation; green: cells permanently arrested prior to anaphase. The asterisk marks cells undergoing cell death. C. The Cdc20-inhibitor proTAME displays similar functional interactions with importin-β as Cdc20-siRNAs. HeLa cells stably expressing mCherryhistone H2B and GFPtubulin were treated with proTAME, importazole, or both, and filmed through mitosis. Experiments were quantified as described. D. Model of microtubule-dependent regulation of APC/C-substrate degradation. Ran-dependent spindle assembly factors are inhibited by importin-β. As seen for HURP, importin-β does not ablate the microtubule-binding of a constitutive motif, the MBD1, indicating that spindle assembly factors might be loaded onto the spindle in their inactive state. RanGTP-dependent dissociation of HURP allows the MBD2 to engage microtubules, thereby activating the spindle assembly factor. Once HURP is not required, its release from microtubules results in rapid ubiquitylation by the APC/C and proteasomal degradation, a reaction that is required for maintaining proper spindle structure and function. See also Figure S6.