Abstract
A versatile method for generating T2-weighting is a T2-preparation (T2-prep) module, which has been used successfully for cardiac imaging at 1.5T. Although it has been applied at 3T, higher fields (B0 ≥ 3T) can degrade B0 and B1 homogeneity and result in non-uniform magnetization preparation. For cardiac imaging, blood flow and cardiac motion may further impair magnetization preparation. In this study, a novel T2-prep module containing multiple adiabatic B1-insensitive refocusing (BIREF-1) pulses is introduced and compared with three previously described modules (a: composite MLEV4, b: modified BIR-4 (mBIR-4), and c: Silver-Hoult–pair). In the static phantom, the proposed module provided similar or better B0 and B1 insensitivity than the other modules. In human subjects (n=21), quantitative measurement of image signal coefficient of variation (CV), reflecting overall image inhomogeneity, was lower for the proposed module (0.10) than for MLEV4 (0.15, p<0.0001), mBIR-4 (0.27, p<0.0001), and Silver-Hoult–pair (0.14, p=0.001) modules. Similarly, qualitative analysis revealed that the proposed module had the best image quality scores and ranking (both, p<0.0001). In conclusion, we present a new T2-preparation module, which is shown to be robust for cardiac imaging at 3T in comparison with existing methods.
Keywords: T2-weighting, cardiac imaging, adiabatic pulses, 3T
INTRODUCTION
T2-weighting is one of the fundamental sources of image contrast in MRI. It is a vital component of coronary magnetic resonance angiography to increase contrast between coronary lumen and myocardium (1). Other cardiovascular applications include the detection of acute ischemic myocardial injury (2) and improved tissue characterization of cardiac masses (3).
A versatile method for generating T2-weighting is a T2-preparation (T2-prep) module (4,5) that leaves the longitudinal magnetization in a state that is dependent on its T2. A typical module consisting of a train of four equally-spaced composite refocusing pulses with Malcolm-Levitt phase cycling (MLEV4) has been widely used at 1.5T (4), but not seen much clinical use at higher fields perhaps since increased B0 and B1 inhomogeneities can result in non-uniform magnetization preparation (6). More recently, T2-prep methods using adiabatic pulses have been explored as an option at higher fields (≥3T). One such method using a modified B1-insensitive rotation (mBIR-4) (7,8) has been shown to be efficacious for fat suppressed, skeletal muscle imaging of the extremities at 3T (8). There is speculation that mBIR-4 may also be useful for coronary MRA (8), but its performance for cardiac applications has not been tested. Another T2-prep module using a pair of adiabatic full passage (AFP) pulses (Silver-Hoult–pair) has been demonstrated to improve image quality for coronary MRA at 3T (6,9-13), but no in vivo comparisons with MLEV4 or any adiabatic modules have been reported.
Higher magnetic field strengths (≥3T) are attractive because the potential gain in signal-to-noise ratio (SNR) allows for reduced scan times and higher resolution images. However, there are significant challenges with high field imaging because of increased B0 and B1 inhomogeneity and specific absorption rate (SAR) (14,15). Additionally, artifacts from cardiac motion and blood flow may be exacerbated at high fields. In this study, a novel T2-prep method containing multiple B1 insensitive refocusing pulses (BIREF-1) was developed to be robust in the presence of cardiac motion, blood flow, and B0 and B1 inhomogeneity. The goal of our study was to systematically compare the performance of our proposed method with existing methods in both static phantoms and in human volunteers to determine the optimal T2-preparation method for clinical cardiac imaging at 3T.
METHODS
Existing T2-prep modules
A standard T2-prep module consists of three components: a tip-down pulse, one or more refocusing pulses, and a tip-up pulse. Three existing methods, MLEV4 (4,5), mBIR-4 (8), and Silver-Hoult–pair (6) are described in Figure 1 a). The refocusing pulses in the MLEV4 approach utilize a phase cycling scheme that reduces the effect of B0 and B1 inhomogeneity (4,16). Additionally, the use of composite refocusing pulses provides second-order corrections to variations in B1 (4). The mBIR-4 module consists of adiabatic pulses that theoretically have improved insensitivity to B1 over composite pulses (17,18), and experimentally this approach has been shown to be relatively B0 insensitive for skeletal muscle imaging (8), but its performance has not been directly compared with the MLEV4 approach. Neither method has been systematically evaluated for human cardiac imaging at 3T. The Silver-Hoult–pair module uses rectangular tip-down and tip-up pulses and a pair of AFP pulses with sech/tanh modulation for refocusing (19). The performance of the Silver-Hoult–pair module has not been previously compared with the mBIR-4 and MLEV4 modules in vivo.
Figure 1. Description of T2-preparation modules.
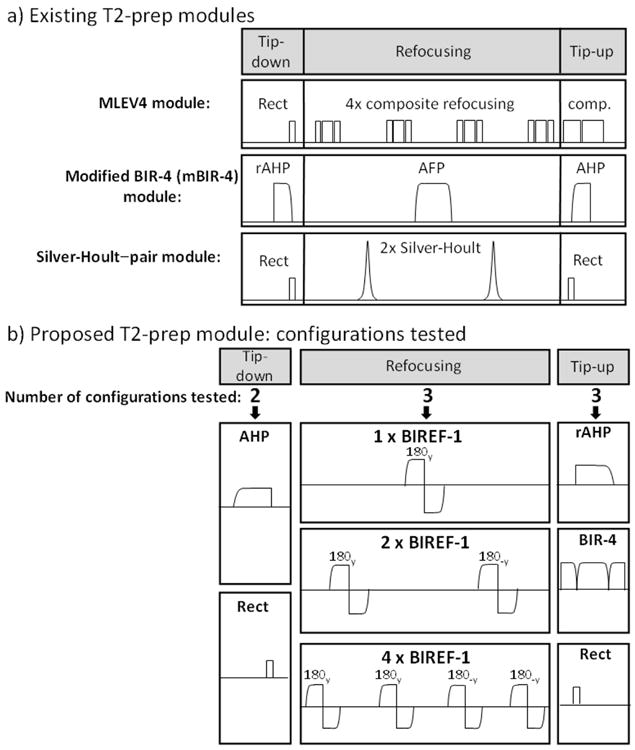
a) Generically, T2-prep modules can be separated into tip-down, refocusing, and tip-up components. Three existing T2-prep modules are shown. The MLEV4 module uses a rectangular (Rect) tip-down pulse (90x), four composite refocusing pulses each consisting of (90x, 180y, 90x), MLEV phase cycling of the refocusing pulses as follows, [180y, 180y, 180-y, 180-y], and a composite tip-up pulse (comp) consisting of (270x, -360x). The mBIR-4 module uses a reverse adiabatic half passage (rAHP) tip-down, an adiabatic full passage (AFP) pulse, and an adiabatic half passage (AHP) tip-up. The Silver-Hoult–pair module uses Rect/Rect tip-down/tip-up pulses and two AFPs with sech/tanh modulation for refocusing. b) The different configurations tested for the proposed T2-prep module are shown. There were two options for tip-down (AHP or Rect), three for refocusing (1, 2 or 4 BIREF-1 pulses), and three for tip-up (AHP, BIR-4, Rect). When multiple BIREF-1 pulses were employed, MLEV phase cycling was used.
Proposed T2-Prep Module
A novel T2-prep module was designed for in vivo cardiac imaging at 3T. The module was constructed to optimally combine B1 insensitivity using adiabatic pulses, and robustness towards motion, flow, and B0 inhomogeneity by using multiple refocusing pulses and MLEV4 phase cycling. The module was implemented on a clinical 3T scanner considering SAR constraints and the expected motion and flow in the human heart.
A primary design consideration was the evaluation of the BIREF-1 refocusing pulse (20) as a major component of the proposed module. BIREF-1, BIR-4 and Silver-Hoult pulses (in pairs) are adiabatic pulses that perform plane rotations, but BIREF-1 requires only two adiabatic half passage (AHP) components, whereas BIR-4 and Silver-Hoult–pair pulses require four. Thus, BIREF-1 has the advantage that more independent refocusing events are possible for a similar energy T2-prep module. A potential disadvantage is worse off-resonance behavior than BIR-4 or Silver-Hoult AFPs (20,21), but this has not been experimentally demonstrated for in vivo imaging.
For optimization of the proposed module, we systematically tested several configurations for each of the three components (see Figure 1 b): (i) number of BIREF-1 refocusing pulses – (1, 2, or 4), (ii) tip-down pulse shape – Rect (rectangular) or AHP, and (iii) tip-up pulse shape – Rect, rAHP (reverse adiabatic half passage), or BIR-4. Tests were performed in phantoms and in vivo and the best performing configuration was directly compared with MLEV4, mBIR-4, and Silver-Hoult–pair modules.
Phantom Experiments
A phantom was made from an Agarose and Nickel Chloride (NiCl2) solution and had three compartments with different T2 and T1 values which were within the range of normal and injured myocardial tissue (22-24) (Figure 2). Phantom images were acquired at 3T (Siemens Verio) using the body coil for both transmit and receive. For all T2-prep modules, the same segmented gradient echo readout was used: TE = 1.58 ms, TR = 3.9 ms, flip angle = 20°, readout bandwidth = 399 Hz/pixel, lines per segment = 15, effective TR (equal to the time between consecutive T2-preps) = 2000 ms, number of averages = 2, FOV = 300 mm, matrix = 320 × 320, in-plane resolution = 0.94 mm × 0.94 mm, and slice thickness = 6 mm. Modules were tested in the phantom using T2-prep times relevant for cardiac imaging (40 ms, 60 ms, and 80 ms).
Figure 2. Phantom setup.
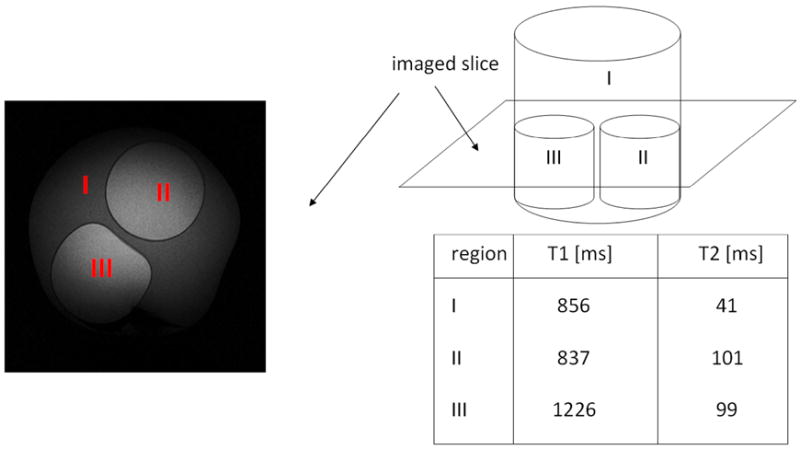
A phantom was constructed out of Agarose gel and NiCl2, with three compartments with different combinations of T2 and T1 values.
For the MLEV4 module, all segments of the composite pulses as well as the Rect tip-down were 800 μs in duration. For the mBIR-4 module, the four segments were based on a tanh/tan modulated AHP with duration of 2.56 ms. The AFP had a frequency sweep of 9.5 kHz. For the Silver-Hoult–pair module, tip-down and tip-up Rect pulses were 800 μs in duration, and hyperbolic secant AFP pulses were 12.8 ms in duration with frequency sweep of 1.6 kHz. For the proposed module, tanh/tan modulated BIREF-1 pulses were used with duration of 5.12 ms and frequency sweep of 9.5 kHz. When multiple BIREF-1 pulses were employed, MLEV phase cycling was used (e.g. for 4 pulses: [180y, 180y, 180-y, 180-y]) (16). For tip-down, Rect (800 μs) or AHP (2.56 ms) pulses were used; for tip-up, Rect, AHP or BIR-4 (similar parameters as the mBIR-4 module without time delays between segments) pulses were used. For all modules, a large spoiling gradient (amplitude = 8 mT/m, duration 6.6 ms) was employed after the tip-up pulse to de-phase any remaining transverse magnetization.
The effect of B0 inhomogeneity on image quality was tested in the phantom by changing the transmitter frequency from -200 Hz to 200 Hz in increments of 100 Hz relative to the water resonance frequency. The B1 sensitivity of the various modules (along with optimal power level) was explored in the phantom by changing the transmitter power to 50%, 75%, 100% and 110% of the calibrated value determined during prescan imaging. For B0 and B1 sensitivity testing, modules were compared using a T2-prep time of 60 ms.
Human Studies
Studies were performed in 21 volunteers without history of cardiac disease (mean age, 45±15 years). Written informed consent was obtained, and the protocol was approved by the Duke Institutional Review Board. Human images were acquired at 3T (Siemens Verio) using the body coil for transmission and phased-array surface coils for receive. Cardiac images were acquired in diastole using a segmented, ECG-gated gradient echo sequence with vendor supplied coil signal intensity correction and representative parameters as follows: TE = 1.66 ms, TR = 4.4 ms, flip angle = 20°, readout bandwidth = 399 Hz/pixel, lines per segment = 23, trigger pulse of 2 RR intervals (RR interval depended on the subject and ranged from 600 ms to 1000 ms), FOV = 360 mm, matrix = 256 × 256, slice thickness = 6 mm.
Investigations
Three different investigations were performed in volunteers. The first focused on the relationship between the number of refocusing pulses in the proposed T2-prep module and image quality. For this investigation (n=4), Rect/Rect tip-down/tip-up pulses were used, and a mid-ventricular short-axis view was acquired using T2-prep times of 40 ms, 60 ms, and 80 ms.
In the second investigation, the best performing configuration of the proposed module (as determined from the phantom studies and the first volunteer investigation) was compared with the three existing modules in 21 volunteers. A mid-ventricular short-axis (n=12) or a four-chamber long-axis (n=9) view was obtained using a T2-prep time of 60 ms.
Finally, for the third investigation, the four modules (the optimized proposed module, mBIR-4, MLEV4, and Silver-Hoult–pair) were compared in one volunteer by obtaining a comprehensive set of images beyond the standard mid-diastolic image with each module. Mid-ventricular short-axis images were obtained using a T2-prep time of 60 ms and with a series of different trigger delays that increased in increments of 75 ms until the dynamic portion of the cardiac cycle (systole and early diastole) was completed. The purpose of this was to directly pin-point the effect of myocardial motion and cavity blood flow–as is encountered through different portions of the cardiac cycle–on image quality.
SAR
All studies in volunteers were performed utilizing strict adherence to FDA guidelines for SAR (<4 W/kg averaged over the whole body for any 15-minute period) and with appropriate RF power safety checks on the clinical scanner. The relative SAR of an RF pulse was determined by numerical integration of the square of the envelope of the pulse shape (25). The overall SAR of a sequence was calculated by summation of the SAR of the individual RF pulses within the sequence. The SAR of each T2-prep sequence was expressed relative to the MLEV4 sequence, which was defined as 100%. Quantitative SAR as calculated by the scanner was 0.56 W/kg for the MLEV4 sequence.
Image Analysis
For the phantom studies, regions of interest (ROIs) were drawn using ImageJ (National Institutes of Health, Bethesda, MD (26)), and the same ROI was used in all images. Image inhomogeneity within the ROI was determined by measuring the coefficient of variation, defined as the standard deviation of signal intensity divided by the mean signal. The average coefficient of variation (CV) of the ROIs was used to determine overall image quality, where larger values indicate more inhomogeneity.
For the human studies, ROIs were drawn in the myocardium and the left ventricular cavity. ROIs were drawn to encompass the majority of the myocardial tissue or cavity blood-pool, respectively, with careful attention to the epicardial and endocardial borders. Image quality was evaluated quantitatively similar to that in phantoms using the coefficient of variation. Additionally, qualitative assessment of image quality was performed by two experienced readers who scored the images on a 4-point scale (3 = poor, 2 = equivocal, 1 = satisfactory, 0 = excellent) based on the following: myocardial homogeneity, cavity homogeneity, and endocardial border definition. Additionally, for each volunteer, images from different T2-prep modules were ranked from best (0) to worst (3) based on overall image quality. To avoid reader bias, the method of T2-preparation and all scan parameters were removed, images were randomly ordered, and were read separately by two readers.
Statistical Analysis
Normally distributed data, such as image inhomogeneity measurements (CV) in the phantom and visual scores in volunteers, were expressed as mean ± SEM (standard error of the mean). Non-normally distributed data, such as CV measurements in volunteers, were expressed as the antilog of the mean and 95% confidence interval of the SEM after initial logarithmic transformation. Generalized linear models (GLM) analysis was used to assess the relationship between the measured CV and the number of refocusing pulses in the proposed T2-prep module for phantom and human studies. The T2-prep time and the number of refocusing pulses were both regarded as repeated measures in the GLM model. For all qualitative measurements, scores from the two readers were averaged since Bland-Altman analysis did not show any significant differences between the readers for image quality (p=0.53) or rank (p=1.00). Analysis of variance (ANOVA) with repeated measures was used for comparisons between different tip-down/tip-up configurations of the proposed module and for comparisons between the best proposed module and previously described modules. Reported p-values for individual pairwise comparisons are after Bonferroni correction. All statistical tests were 2-tailed; values of p<0.05 were regarded as significant.
RESULTS
Phantom Studies
T2-prep Optimization
The relationship between the number of refocusing pulses and image quality for the proposed T2-prep module with Rect/Rect tip-down/tip-up is shown in Figure 3 a). For all 3 T2-prep times (40 ms, 60 ms, 80 ms) more refocusing pulses improved image homogeneity (p<0.001). For instance, with a T2-prep time of 60 ms the coefficient of variation was reduced to 60% of its starting value going from 1 to 4 refocusing pulses (0.58 to 0.35). The MLEV4 module performed similar to the proposed module with 1 refocusing pulse, whereas the mBIR-4 and Silver-Hoult–pair modules were comparable to the proposed module with 4 refocusing pulses. Typical images are shown in Figure 3 b).
Figure 3. The effect of the number of refocusing pulses.
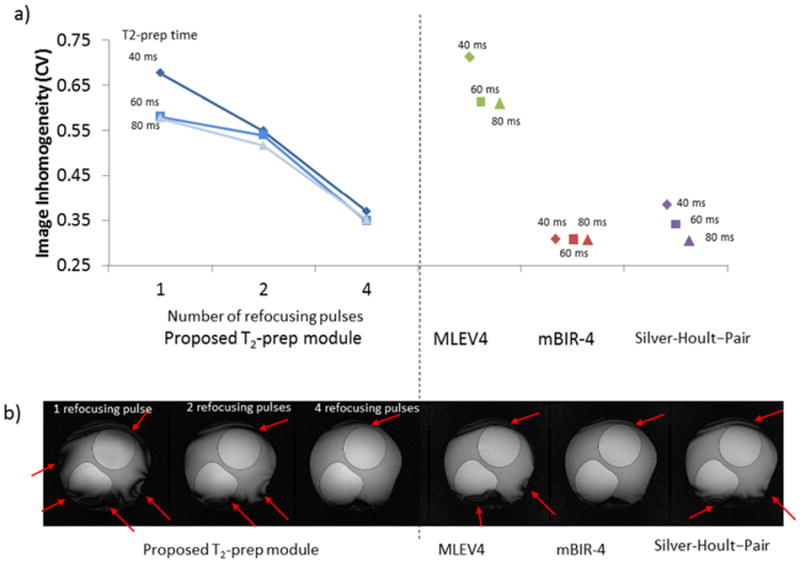
a) Left portion of the graph displays image inhomogeneity (coefficient of variation) versus number of refocusing pulses for the proposed T2-prep module with Rect/Rect tip-down/tip-up pulses. For all T2-prep times, more refocusing pulses improved image homogeneity (ie reduced coefficient of variation). The performance of MLEV4, mBIR-4 and Silver-Hoult–pair modules are shown on the right portion of the graph for comparison. The MLEV4 module had a relatively high level of inhomogeneity. b) Typical images with each module for a T2-prep time of 40 ms. Arrows show areas of inhomogeneity.
Other combinations of tip-down/tip-up pulses were tested for the proposed module with 4 refocusing pulses. The coefficient of variation (averaged from the 3 tested T2-prep times) for the various configurations are shown in Table 1. The Rect/Rect configuration had the lowest CV and had superior performance compared with the Rect/BIR-4 configuration (p=0.007). Although the difference in image quality did not reach statistical significance in comparison with the other tip-down/tip-up combinations, the Rect/Rect configuration was chosen for further testing given its advantages in terms of simple implementation, shorter duration, and lower SAR.
Table 1.
Comparison of Tip-down and Tip-up Configurations of the Proposed T2-prep Module.
| Module Configurations | Image Inhomogeneity (CV± SEM) | P-value (comparison with first configuration) | ||
|---|---|---|---|---|
| Tip-Down | Tip-Up | Number of Refocusing Pulses | ||
| Rect | Rect | 4 | 0.357 ± 0.006 | -- |
| Rect | rAHP | 4 | 0.403 ± 0.025 | 0.150 |
| Rect | BIR-4 | 4 | 0.416 ± 0.035 | 0.007 |
| AHP | Rect | 4 | 0.413 ± 0.007 | 0.068 |
| AHP | rAHP | 4 | 0.422 ± 0.023 | 0.111 |
Sensitivity to B1 and B0
The optimized configuration of the proposed T2-prep module (4 BIREF-1 refocusing pulses with Rect/Rect tip-down/tip-up), demonstrated similar B1 sensitivity compared with the mBIR-4 module and reduced sensitivity compared with the MLEV4 and Silver-Hoult–pair modules (Figure 4). With a 25% decrease in B1 amplitude, both the proposed and mBIR-4 modules had mild increases in image inhomogeneity (26% and 23% increase in CV, respectively), whereas the MLEV4 and the Silver-Hoult–pair modules had more marked increases in inhomogeneity (63% and 68% increase in CV, respectively).
Figure 4. B1 Sensitivity.
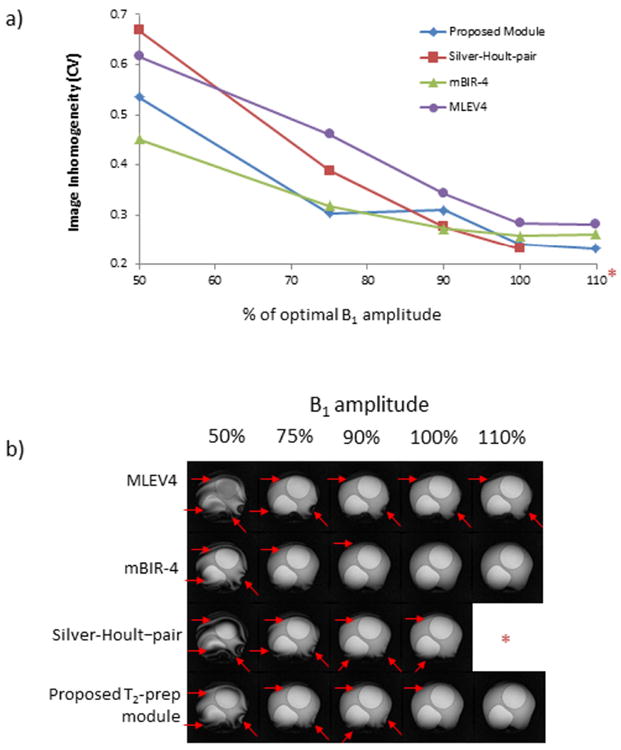
a) B1 amplitude was varied between 50% to 110% of the calibrated value for the tested modules (*Note that B1 amplitude at 110% could not be tested for the Silver-Hoult–pair module because peak amplitude exceeded scanner capability). T2-prep time was 60 ms for all modules, and the coefficient of variation (CV) is shown as a function of transmitter voltage. b) Typical images are shown. Red arrows identify regional inhomogeneities. The mBIR-4 and the proposed T2-prep module provided a larger range of insensitivity to B1 than the MLEV4 and Silver-Hoult–pair modules.
The proposed, mBIR-4 and Silver-Hoult-pair modules had reduced sensitivity to B0 compared with the MLEV4 module (Figure 5). They performed well over a range of ±200 Hz, while MLEV4 only performed well on-resonance.
Figure 5. B0 sensitivity.
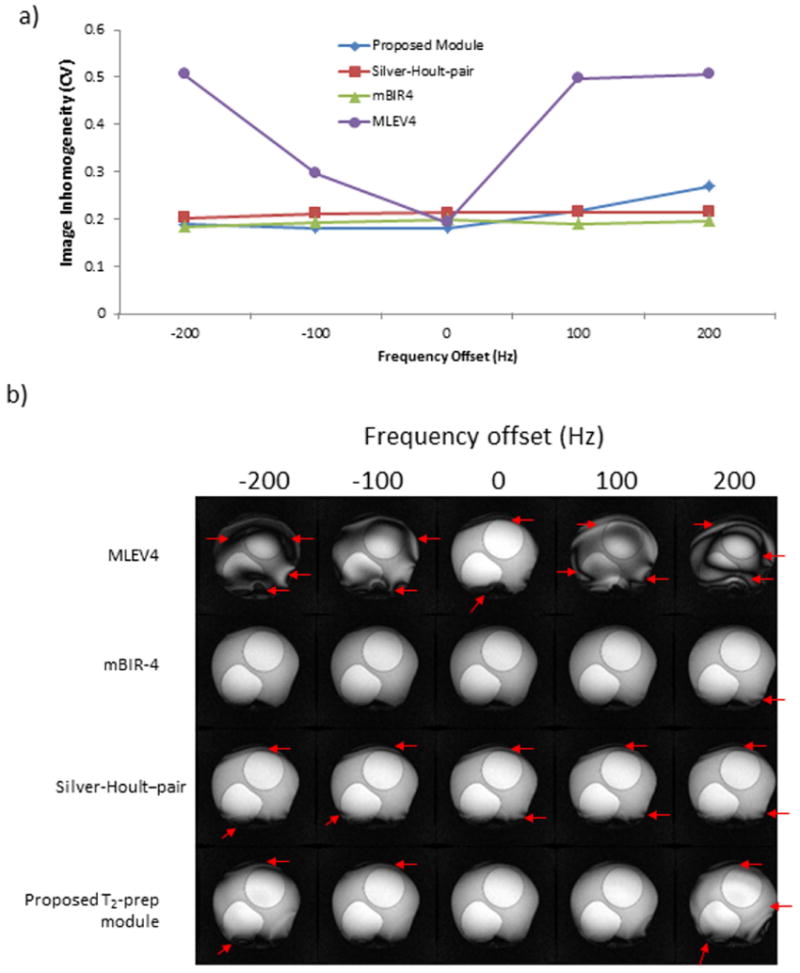
a) Transmitter frequency was varied from -200 Hz to +200 Hz for the tested modules. T2-prep time was 60 ms for all modules, and the coefficient of variation (CV) is shown as a function of frequency offset. b) Typical images are shown. Red arrows point out areas of image inhomogeneity. The mBIR-4, Silver-Hoult-pair and proposed T2-prep modules were relatively insensitive to changes in B0 unlike the MLEV4 module.
Human Studies
Number of Refocusing Pulses
Similar to the phantom results, more refocusing pulses improved image homogeneity for the proposed T2-prep module in vivo, although improvements were generally greater (Figure 6 a). For myocardial tissue, the coefficient of variation dropped to 53% of its starting value going from 1 to 4 refocusing pulses (0.51 to 0.27, p<0.0001). In the LV cavity, the coefficient of variation was reduced to 28% of its starting value (0.39 to 0.11, p<0.0001). Representative images in one volunteer are shown in Figure 6 b) and demonstrate the effect of more refocusing pulses, especially in the LV cavity.
Figure 6. Effect of the number of refocusing pulses in the proposed T2-prep module in human volunteers.
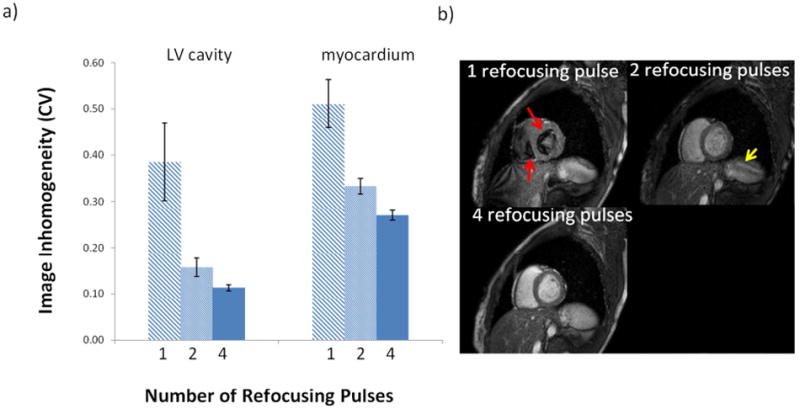
a) The coefficient of variation in the LV cavity and myocardium averaged for all T2-prep times (40, 60, and 80 ms) in 4 subjects decreased with increasing number of refocusing pulses (p<0.0001 for both). b) Typical images from one volunteer (T2-prep time was 60 ms). With 1 refocusing pulse, there are significant artifacts due to motion and flow in the left and right ventricular cavities (red arrows point to signal voids). Signal void artifacts are mostly gone with 2 refocusing pulses, but the overall signal intensity is low and banding is still seen (yellow arrow). With 4 refocusing pulses, LV cavity signal is bright and homogeneous, and the myocardium is uniform.
Quantitative and Qualitative Comparison of T2-prep Modules
The performance of four T2-prep modules (the optimized proposed module, mBIR-4, MLEV4, and Silver-Hoult–pair) were compared in 21 volunteers. Quantitative analysis (Table 2) demonstrated that the proposed module had the lowest coefficient of variation for LV cavity (0.07), LV myocardium (0.14) and overall (0.10). This reached statistical significance in comparison with each of the existing modules (all, p≤0.001). Similarly, qualitative analysis (Table 3) revealed that the proposed module had the best quality scores and ranking (all, p<0.0001). The Silver-Hoult–pair and MLEV4 modules consistently placed second and third, respectively, on both quality scores and rank. The mBIR-4 module performed poorly on both quantitative and qualitative measures, primarily due to severe inhomogeneity of the LV cavity blood-pool, which had a coefficient of variation that on average was more than twice that of the other techniques.
Table 2.
Comparison of Image Inhomogeneity in Volunteers
| LV Cavity | LV Myocardium | Overall | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| T2 Prep module | Mean CV* (n = 21) | P-value (vs proposed module) | Mean CV* (n = 21) | P-value (vs proposed module) | Mean CV* (n = 42) | P-value (vs proposed module) |
| Proposed Module | 0.07 (0.06 – 0.08) | -- | 0.14 (0.13 – 0.16) | -- | 0.10 (0.09 – 0.11) | -- |
| Silver-Hoult–pair | 0.11 (0.09 – 0.13) | 0.0005 | 0.19 (0.17 – 0.22) | 0.003 | 0.14 (0.13 – 0.17) | 0.001 |
| MLEV-4 | 0.11 (0.09 – 0.13) | <0.0001 | 0.22 (0.19-0.25) | 0.006 | 0.15 (0.13 – 0.18) | <0.0001 |
| mBIR-4 | 0.23 (0.18 – 0.30) | <0.0001 | 0.32 (0.27 – 0.38) | <0.0001 | 0.27 (0.23 – 0.32) | <0.0001 |
Indices reported as the antilog of the mean CV (95% confidence interval of the SEM) of log transformed data.
Table 3.
Comparison of Image Quality Scores and Ranks in Volunteers
| T2 Prep module (n = 21) | Mean Rank ± SEM (lower is better) | P-value (vs proposed module) | Mean Visual Score ± SEM (lower is better) | P-value (vs proposed module) |
|---|---|---|---|---|
| Proposed Module | 1.17 ± 0.07 | -- | 0.48 ± 0.13 | -- |
| Silver-Hoult–pair | 2.09 ± 0.13 | <0.0001 | 1.21 ± 0.14 | <0.0001 |
| MLEV-4 | 2.81 ± 0.15 | <0.0001 | 1.86 ± 0.12 | <0.0001 |
| mBIR-4 | 3.92 ± 0.15 | <0.0001 | 2.74 ± 0.18 | <0.0001 |
In one volunteer, multiple images were acquired at different time points in the cardiac cycle (Figure 7). The proposed module provided uniform tissue and blood preparation throughout the cardiac cycle. The Silver-Hoult–pair provided mostly uniform contrast, although there were some flow artifacts during early systole. The MLEV4 module showed moderate sized regions of signal loss at multiple time points. The mBIR-4 module performed poorly and had heterogeneous signal in both cavity and myocardium at every phase of the cardiac cycle that was imaged.
Figure 7. Images acquired at different phases of the cardiac cycle.
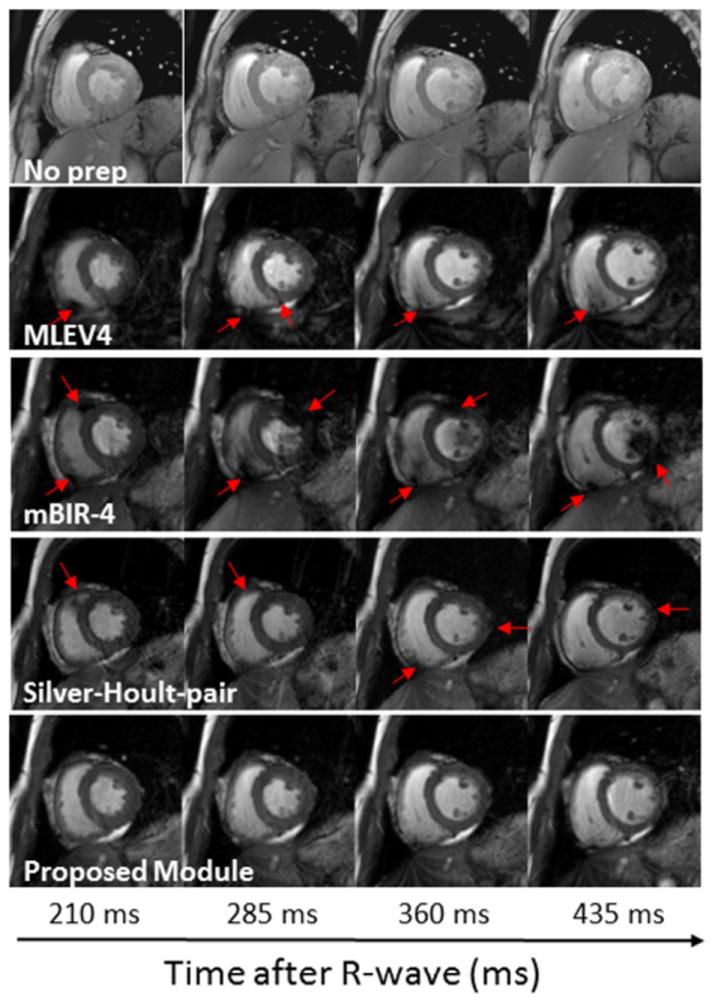
Images were acquired with incremental changes in the trigger delay (75 ms step size) using a T2-prep time of 60 ms for those with T2-prep modules. The top row shows images acquired without a T2-prep to show the difference in myocardial contrast. The horizontal axis represents the time from the R-wave to the center of k-space for the images. Regions of inhomogeneity are indicated with red arrows. The proposed module provided the most uniform tissue and blood preparation throughout the cardiac cycle of the four T2-prep modules that were tested.
SAR
In all volunteers, images with the four T2-prep sequences (MLEV4, mBIR-4, Silver-Hoult-pair, proposed module) were obtained without exceeding any SAR constraints. The relative SAR of the T2-prep sequences incorporating MLEV4, mBIR-4, Silver-Hoult–pair, and proposed modules were 100%, 155%, 139%, and 252%, respectively. If one considers each module in terms of SAR per independent refocusing event, the results were as follows: MLEV4 = 25%, mBIR-4 = 155%, Silver-Hoult–pair = 139%, proposed module = 63%.
DISCUSSION
We have proposed and implemented a new T2-preparation module for cardiac imaging at 3T. The proposed module was validated in both phantom and human volunteer studies, and in comparison with existing T2-prep methods, was shown to be robust in the presence of cardiac motion and blood flow as well as B0 and B1 inhomogeneities.
Performance of existing T2-prep modules for cardiac imaging at 3T
The MLEV4 and mBIR-4 T2-prep modules have not been previously assessed for clinical cardiac imaging at 3T, although there is speculation regarding their potential utility (6,8). Several studies have reported the efficacy of the Silver-Hoult–pair module for coronary MRA at 3T (6,9-13), however, head-to-head comparisons with other clinically used T2-prep methods have not been described.
The MLEV4 module was the most sensitive to changes in B0 and B1 as highlighted in the static phantom experiments. The range of B0 offsets for which MLEV4 provided uniform magnetization preparation was less than half that for modules using adiabatic pulses. Similarly, the range of B1 amplitudes over which MLEV4 provided homogeneous magnetization was more limited than with the other modules. In human volunteers, cardiac images were often unsatisfactory. These findings are consistent with previous numerical simulations which suggested, given the expected B0 and B1 field variations for clinical imaging at 3T, the performance of MLEV4 would be suboptimal (6).
The mBIR-4 module was less sensitive to B0 and B1 than MLEV4 in the static phantom, but its exceptional sensitivity to cardiac motion and flow resulted in poor performance in human volunteers. The artifacts due to flow were dramatic – images acquired with this module often had large signal voids in the LV and RV cavity as if incomplete black-blood preparation had been attempted. Although the description of mBIR-4 reported excellent performance for T2-weighted imaging of peripheral skeletal muscle (8), an inherent limitation is that signal is only refocused if there is precise phase compensation from all components of the module, including tip-down and tip-up. Thus, motion-related phase shifts occurring at any time throughout the entire period of the T2-prep module will preclude complete refocusing and result in signal loss and artifacts. Despite conjecture that mBIR-4 may be useful for coronary MRA (8), our findings indicate that this module is a poor choice for cardiac imaging at 3T.
The Silver-Hoult–pair module performed slightly inferior to mBIR-4 in the static phantom, but image quality was distinctly better in human volunteers. Quantitative analysis suggested a minor improvement over the MLEV4 module, but greater differences were noted on the visual scores. In agreement with prior reports (6,9-13), image quality was satisfactory or better in nearly all volunteers. Accordingly, visual ranking was consistently high at second, just behind the proposed module.
Proposed T2-prep module
In the static phantom, the proposed module required 4 BIREF-1 pulses to provide the same level of image homogeneity as the mBIR-4 or Silver-Hoult–pair modules. Thus, when considering individual adiabatic pulses, BIREF-1 appears to have worse performance. This is potentially explained by the fact that the BIREF-1 pulse has asymmetric off-resonance behavior and simulations have described an off-resonance singularity (20). Nonetheless, the proposed module with 4 BIREF-1 pulses provided similar B0 and B1 insensitivity as the mBIR-4 and Silver-Hoult–pair modules in phantoms, and in vivo, image quality was superior. Specifically, in human subjects, overall image inhomogeneity was reduced in comparison with each of the existing modules (all p≤0.001, Table 2). The proposed module also performed well on qualitative analysis, receiving the best rank and visual scores (both, p<0.0001). These results demonstrate that for in vivo cardiac imaging at 3T, after a sufficient level of B0 and B1 insensitivity is reached by the T2-prep module, adverse effects from cardiac motion and blood flow predominate. Attention, therefore, should be placed on reducing these effects rather than on further improvements in B0 and B1 insensitivity for uniform magnetization preparation.
Four BIREF-1 pulses were at the core of the new module, which proved sufficient to prevent motion related artifacts for cardiac imaging in healthy volunteers. Similar to fast-spin echo, where shortening the echo spacing reduces flow and motion induced signal loss (5), increasing the number of refocusing pulses for a given T2-prep time improves insensitivity to motion by effectively reducing the inter-pulse spacing. Despite no flow sensitizing gradients in the T2-prep module, motion-related phase shifts occur since moving spins sample the inherent spatial variation in B0. Thus, decreased time between refocusing events equates with shorter distances traveled for moving spins, fewer B0 environments sampled, and less signal loss (5). We chose the BIREF-1 pulse since it would allow more independent refocusing events without exceeding SAR limitations. Each BIREF-1 pulse, by itself is a true refocusing pulse, where the second half of the pulse immediately compensates the phase dispersion created by the first half without any delay. Thus, unlike the Silver-Hoult pulse (21,27), it need not be applied in pairs, and it requires only 2 AHPs per independent refocusing event rather than 4 for Silver-Hoult or 4 for other common adiabatic pulses such as the BIR-4 pulse. To the best of our knowledge, the current study represents the first clinical application of the BIREF-1 pulse since its original description (20).
The shape of tip-down/tip-up pulses was also explored. Prior studies have used Rect/Rect (6), Rect/Composite (4), or rAHP/AHP pulses (8). Simulations have suggested that a Rect/Composite combination can improve off-resonance performance in comparison with Rect/Rect (4), however, we are unaware of experimental data from direct head-to-head comparisons between these or any other tip-down/tip-up combinations. Our results in phantoms indicate that Rect pulses perform as well or better than AHP pulses, and this is of practical relevance since Rect pulses are shorter and require less power. In agreement with results found by Nezafat et al. (6), we show that Rect/Rect pulses can be implemented as part of a successful T2-prep for in vivo cardiac imaging at 3T.
Limitations
Although the relative SAR per independent refocusing event was lower for the proposed module than the other adiabatic T2-prep modules, the four refocusing events resulted in the highest total SAR. This could become an issue if steady-state free-precession (SSFP) readout is desired, rather than spoiled gradient-echo, as SAR limits may be reached because of the higher flip angles. However, SSFP imaging at 3T is often problematic, given its sensitivity to B0 inhomogeneity and the development of banding artifacts and pulsatile flow artifacts (14). Therefore, gradient-echo imaging is common for cardiac imaging at 3T, and from a practical clinical perspective, it should be noted that the proposed sequence as tested with gradient-echo readout ran in all volunteers without exceeding any SAR constraints.
SAR restrictions could become a limiting factor if more than four refocusing pulses are desired. While high quality T2-prep images were obtained in vivo in the current investigation, these were of normal volunteers in which cardiac blood flow velocities likely peak at 1-2 meters per second (29). For patients with cardiac disease such as mitral stenosis or aortic insufficiency, higher velocities up to 4 m/s are commonplace (29) and it is possible that four refocusing pulses may be insufficient. Likewise, SAR could become an issue if T2-prep modules are played every heartbeat, rather than every two heartbeats. In both situations, the SAR per BIREF-1 pulse may need to be reduced. This could be accomplished by changing the parameters of the pulse. For example, increasing pulse duration by 50% (5.1 ms to 7.6 ms) while scaling down pulse amplitude to maintain the same total area of B1 would lead to a 56% reduction in SAR. Unfortunately, this would also lead to a moderate (33%) reduction in frequency sweep (9.5 kHz to 6.3 kHz), which could diminish B0 insensitivity. Whether this would adversely affect image quality in vivo would need to be examined in future studies, however, one of the main findings of the current study is that T2-prep performance in phantoms is quite distinct from its performance in vivo. For the latter, it appears that adverse effects from cardiac motion and blood flow predominate, and consequently, the accommodation of a larger number of refocusing pulses may be more important than a slight worsening in the B0 or B1 insensitivity of each of the refocusing pulses.
As an additional consideration, we note that in the presence of RF irradiation, relaxation of magnetization aligned perpendicular to the effective magnetic field is characterized by time constant T2ρ and may lead to an apparent reduction in T2-prep time (28). Finally, for cardiac imaging, fat signal suppression is often desired along with T2-weighting. In the current study, T2-prep modules were not examined in combination with fat saturation, but this should be considered for future investigations.
Summary
In this study, a new T2-prep module is proposed and validated. The module was shown to provide improved image quality in comparison with existing T2-prep methods for cardiac imaging at 3T.
Acknowledgments
Funding for the research was provided in part by National Institutes of Health grant 2RO1-HL064726 (RJK).
References
- 1.Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation. 1999;99(24):3139–3148. doi: 10.1161/01.cir.99.24.3139. [DOI] [PubMed] [Google Scholar]
- 2.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magnetic Resonance in Medicine. 2007;57(5):891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radiographics. 2005;25(5):1255–1276. doi: 10.1148/rg.255045721. [DOI] [PubMed] [Google Scholar]
- 4.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary Angiography with Magnetization-Prepared T2 Contrast. Magnetic Resonance in Medicine. 1995;33(5):689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 5.Wright GA, Nishimura DG, Macovski A. Flow-independent magnetic resonance projection angiography. Magnetic Resonance in Medicine. 1991;17(1):126–140. doi: 10.1002/mrm.1910170117. [DOI] [PubMed] [Google Scholar]
- 6.Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI. B-1-insensitive T-2 preparation for improved coronary magnetic resonance angiography at 3 T. Magnetic Resonance in Medicine. 2006;55(4):858–864. doi: 10.1002/mrm.20835. [DOI] [PubMed] [Google Scholar]
- 7.Garwood M, Ke Y. Symmetric pulses to induce arbitrary flip angles with compensation for rf inhomogeneity and resonance offsets. Journal of Magnetic Resonance (1969) 1991;94(3):511–525. [Google Scholar]
- 8.Nezafat R, Ouwerkerk R, Derbyshire AJ, Stuber M, McVeigh ER. Spectrally Selective B-1-Insensitive T-2 Magnetization Preparation Sequence. Magnetic Resonance in Medicine. 2009;61(6):1326–1335. doi: 10.1002/mrm.21742. [DOI] [PubMed] [Google Scholar]
- 9.Wieben O, Francois C, Reeder SB. Cardiac MRI of ischemic heart disease at 3 T: Potential and challenges. European Journal of Radiology. 2008;65(1):15–28. doi: 10.1016/j.ejrad.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Xie JS, Lai P, Bhat H, Li DB. Whole-Heart Coronary Magnetic Resonance Angiography at 3.0T Using Short-TR Steady-State Free Precession, Vastly Undersampled Isotropic Projection Reconstruction. Journal of Magnetic Resonance Imaging. 2010;31(5):1230–1235. doi: 10.1002/jmri.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Agarwal H, Stuber M, Schar M. Practical Signal-to-Noise Ratio Quantification for Sensitivity Encoding: Application to Coronary MR Angiography. Journal of Magnetic Resonance Imaging. 2011;33(6):1330–1340. doi: 10.1002/jmri.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Paetsch I, Schnackenburg B, Fleck E, Weiss RG, Stuber M, Jahnke C. Use of 2D Sensitivity Encoding for Slow-Infusion Contrast-Enhanced Isotropic 3-T Whole-Heart Coronary MR Angiography. American Journal of Roentgenology. 2011;197(2):374–382. doi: 10.2214/AJR.10.5724. [DOI] [PubMed] [Google Scholar]
- 13.Gharib AM, Abd-Elmoniem KZ, Herzka DA, Ho VB, Locklin J, Tzatha E, Stuber M, Pettigrew RI. Optimization of coronary whole-heart MRA free-breathing technique at 3 Tesla. Magnetic Resonance Imaging. 2011;29(8):1125–1130. doi: 10.1016/j.mri.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein MA, Huston J, Ward HA. Imaging artifacts at 3.0T. Journal of Magnetic Resonance Imaging. 2006;24(4):735–746. doi: 10.1002/jmri.20698. [DOI] [PubMed] [Google Scholar]
- 15.Gutberlet M, Noeske R, Schwinge K, Freyhardt P, Felix R, Niendorf T. Comprehensive Cardiac Magnetic Resonance Imaging at 3.0 Tesla: Feasibility and Implications for Clinical Applications. Investigative Radiology. 2006;41(2):154–167. doi: 10.1097/01.rli.0000195840.50230.10. [DOI] [PubMed] [Google Scholar]
- 16.Levitt MH, Freeman R, Frenkiel T. Broadband heteronuclear decoupling. Journal of Magnetic Resonance (1969) 1982;47(2):328–330. [Google Scholar]
- 17.Baum J, Tycko R, Pines A. Broadband and Adiabatic Inversion of a Two-Level System by Phase Modulated Pulses. Physical Review A. 1985;32(6):3435–3447. doi: 10.1103/physreva.32.3435. [DOI] [PubMed] [Google Scholar]
- 18.Hardy CJ, Edelstein WA, Vatis D. Efficient adiabatic fast passage for NMR population inversion in the presence of radiofrequency field inhomogeneity and frequency offsets. Journal of Magnetic Resonance (1969) 1986;66(3):470–482. [Google Scholar]
- 19.Silver MS, Joseph RI, Hoult DI. Highly selective pi/2 and pi-pulse generation. Journal of Magnetic Resonance. 1984;59(2):347–351. [Google Scholar]
- 20.Ugurbil K, Garwood M, Rath AR, Bendall MR. Amplitude and Frequency/Phase-Modulated Refocusing Pulses that Induce Plane Rotations Even in the Presence of Inhomogeneous B1 fields. Journal of Magnetic Resonance. 1988;78:472–497. [Google Scholar]
- 21.Garwood M, DelaBarre L. The return of the frequency sweep: Designing adiabatic pulses for contemporary NMR. Journal of Magnetic Resonance. 2001;153(2):155–177. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- 22.Kraft KA, Fatouros PP, Clarke GD, Kishore PRS. An MRI phantom material for quantitative relaxometry. Magnetic Resonance in Medicine. 1987;5(6):555–562. doi: 10.1002/mrm.1910050606. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell MD, Kundel HL, Axel L, Joseph PM. Agarose as a tissue equivalent phantom material for NMR imaging. Magnetic Resonance Imaging. 1986;4(3):263–266. doi: 10.1016/0730-725x(86)91068-4. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P, Socolow J, Patel S, Pettigrew RI, Oshinski JN. Effect of Gd-DTPA-BMA on blood and myocardial T1 at 1.5T and 3T in humans. Journal of Magnetic Resonance Imaging. 2006;23(3):323–330. doi: 10.1002/jmri.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haacke C. Magnetic resonance imaging : physical principles and sequence design. New York: Wiley-Liss; 1999. [Google Scholar]
- 26.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 27.Conolly S, Glover G, Nishimura D, Macovski A. A reduced power selective adiabatic spin-echo pulse sequence. Magnetic Resonance in Medicine. 1991;18(1):28–38. doi: 10.1002/mrm.1910180105. [DOI] [PubMed] [Google Scholar]
- 28.Michaeli S, Sorce DJ, Garwood M. T-2 rho and T-1 rho adiabatic relaxations and contrasts. Current Analytical Chemistry. 2008;4(1):8–25. [Google Scholar]
- 29.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. European Journal of Echocardiography. 2009;10(1):1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]


