Abstract
Burkholderia cepacia, a Gram-negative bacterium commonly found in water and soil, is a rare cause of endophthalmitis. The authors report a case of a penicillin-allergic patient who presented 15 days after an uneventful injection of ranibizumab for neovascular age-related macular degeneration with culture-positive B cepacia endophthalmitis. Initial antibiotic therapy using non-penicillin-based medications was not successful in eradicating the bacteria. Subsequent treatment with a third-generation cephalosporin resulted in complete resolution of the infection. B cepacia should be included among the bacterial species that may cause endophthalmitis after intravitreal injections.
Background
Exogenous endophthalmitis following intravitreal injections is uncommon, but a significant concern as the number of injections increases. Most culture-positive cases are due to normal conjunctival or skin flora.1 Burkholderia cepacia is a Gram-negative bacterium which is a very unusual cause of endophthalmitis, representing 1.8% of cases.2 It has been isolated following a variety of intraocular procedures and penetrating ocular trauma. B cepacia is resistant to many antibiotics, but often sensitive to cephalosporins. Penicillin allergies are reported in 10% of patients, although the rate of confirmed reactions and cephalosporin cross-reactivity is much lower.3 This is the first case of B cepacia endophthalmitis reported following an in-office intravitreal injection of ranibizumab (Luncentis; Novartis). That our patient also had penicillin allergy created a treatment dilemma.
Case presentation
A 91-year-old woman with a penicillin allergy (severe rash) and with an ocular history of bilateral pseudophakia presented with a 4-day history of painless decreased vision and floaters in the right eye, which began 11 days after a maintenance intravitreal injection of ranibizumab for neovascular AMD. Our standard protocol for intravitreal injections, includes instillation of 5% povidone iodine to the ocular fornix, sterile speculum and calipers, silence and surgeon use of a surgical mask. On presentation, her best corrected visual acuity was 20/60 in the right eye, which had decreased from 20/40 preinjection. Slit-lamp examination demonstrated an absence of conjunctival injection, 2+ anterior chamber cells and keratic precipitates without a hypopyon. Dilated fundus examination revealed 3+ vitreous cells and haze with an attached retina (figure 1A). B-scan ultrasonography was performed and confirmed the fundus findings (figure 1B).
Figure 1.
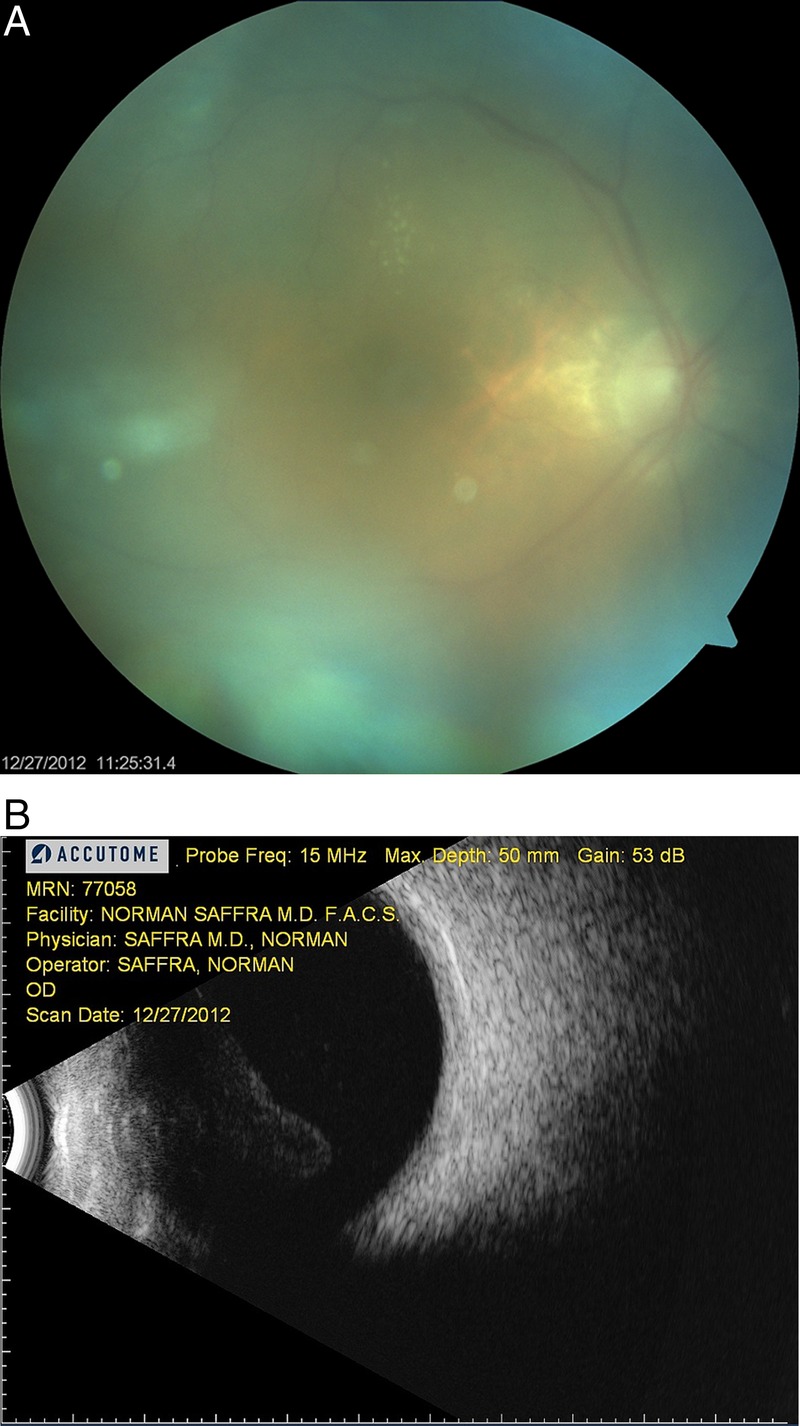
(A) Fundus photograph and (B) corresponding B-scan ultrasonography demonstrating diffuse vitritis with an attached retina.
Investigations
A clinical diagnosis of postprocedure exogenous endophthalmitis was suspected. As per the Endophthalmitis Vitrectomy Study (EVS), based on her visual acuity, a vitreous tap and injection was recommended. The patient however, was very anxious and refused to give consent, preferring an operative procedure with parenteral sedation. A pars plana vitrectomy was therefore performed on the day of presentation and an undiluted vitreous biopsy was sent for culture. Culture analysis was available 7 days after the vitrectomy and revealed B cepacia, which was sensitive to ceftazidime and levofloxacin, and resistant to amikacin, ampicillin, cefazolin, cefotaxime and nitrofurantoin.
Treatment
At the time of the vitrectomy, empiric intravitreal injections of vancomycin (1 mg/0.1 mL), amikacin (400 mcg/0.1 mL), and dexamethasone (400 mcg/0.1 mL), were given in light of the patient's penicillin allergy. Her postoperative regimen included difluprednate 0.05%, bromfenac 0.09% and loteprednol etabonate 0.5%.
Outcome and follow-up
Postoperatively, the patient's vitritis improved, did not resolve completely. When the vitreous biopsy culture results were available, an infectious diseases consult was obtained, and the decision was made to give an intravitreal injection of ceftazidime (2.25 mg/0.1 mL). Post injection, the patient's inflammation completely resolved and visual acuity returned to her baseline, without any systemic sequelae. One year postoperative visual acuity is 20/30, but she continues to require antivascular endothelial growth factor (VEGF) treatment for a choriodal neovascular membrane (figure 2).
Figure 2.
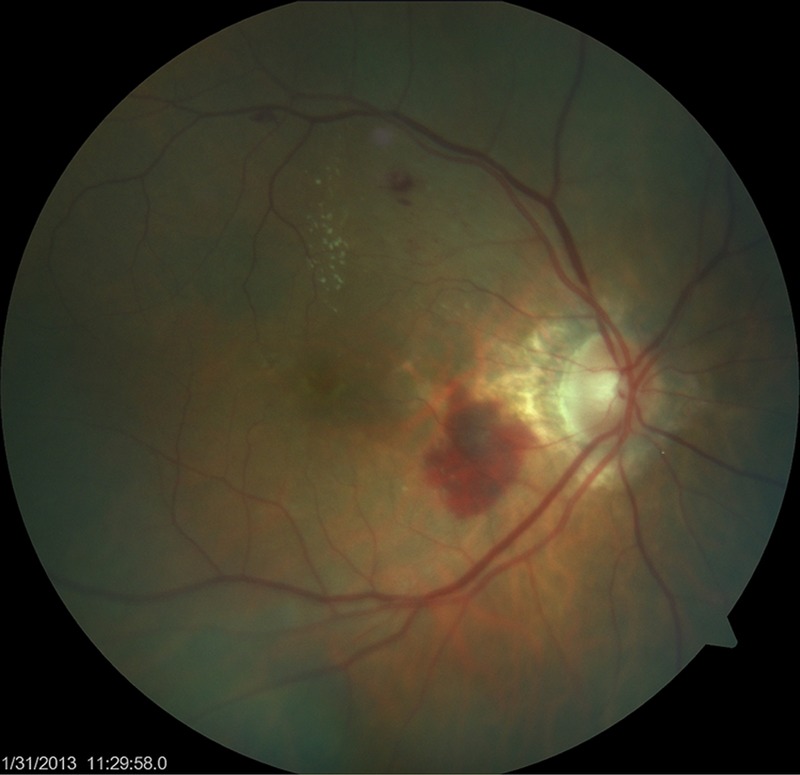
Fundus photograph taken 1 month postvitrectomy, demonstrating a complete resolution of the endophthalmitis but persistence of the original choroidal neovascular membrane.
Discussion
B cepacia is a widespread organism found in water and soil and has been implicated in several nosocomial outbreaks due to its ability to survive in the antiseptics chlorhexidine and povidone iodine.4 5 It is also a frequent cause of pneumonia in immunocompromised patients with lung disease, such as cystic fibrosis. Our patient denied a history of lung disease or recent hospitalisations. She did live in an area devastated by Superstorm Sandy, which caused widespread water damage 6 weeks prior to her ranibizumab injection, providing one possible aetiology for her exposure.
Endophthalmitis following intravitreal injection of anti-VEGF agents is rare, occurring in 0.02–0.21% of injections.6–8 The overwhelming majority of culture-positive cases (92.8%) are caused by Gram-positive commensal organisms, with a predominance of Streptococcal and Staphylococcal species.8 9 While several cases of B cepacia exogenous endophthalmitis have been reported, to our knowledge this is the first case occurring after an intravitreal injection of an anti-VEGF agent (MEDLINE search).
The EVS guidelines recommend vitrectomy for light perception vision or worse in cases of acute postoperative endophthalmitis after cataract surgery.10 The intravitreal injections used in this landmark study included vancomycin and amikacin. Currently, vancomycin and ceftazidime are more frequently used, with amikacin playing a larger role in patients with a penicillin allergy. The role of intravitreal steroids in treating endophthalmitis remains controversial. Level one evidence for the treatment of endophthalmitis that does not fall into the strict entry criteria of the EVS study is limited.
While our standard protocol for endophthalmitis treatment includes intravitreal injections of vancomycin and ceftazidime, our patient reported a history of penicillin allergy, so empiric treatment with ceftazidime was replaced with amikacin. While this is an appropriate management in the majority of cases, the organism in this case was resistant to aminoglycosides. B cepacia produces lipopolysaccharide and β-lactamase, making it resistant to many antibiotics.11 Obviously, there is concern for using cephalosporin in a patient with a known penicillin allergy, but the cross-reactivity rate between penicillin and cephalosporins are generally low.3 After speciation and sensitivities became available, an injection of ceftazidime was given without adverse effects. While oral levofloxacin has been reported to achieve mean inhibitory concentrations in the vitreous against many organisms, its role in the treatment of endothalmitis has not been established.12 Previous case reports demonstrate a wide spectrum of patient outcomes associated with B cepacia.2 13 Our patient did well despite the delay in administering the appropriate antibiotics, possibly because the early vitrectomy reduced the bacterial load.
The number of anti-VEGF intravitreal injections has risen rapidly over the past few years, and will likely continue to climb. This case illustrates the importance of considering uncommon organisms in patients with unusual presentations of exogenous endophthalmitis after intravitreal injection.
Learning points.
Consider uncommon organisms in patients with an unusual presentation of exogenous endophthalmitis after intravitreal injection.
When treating endophthalmitis in penicillin-allergic patients, first-line therapy should use non-penicillin-based medications.
In circumstances where they are required, cross-reactivity is low and penicillin-based antibiotics can be used with caution in patients without life-threatening allergies.
Footnotes
Contributors: NS and EM contributed equally to the analysis of the data and the preparation of the manuscript. It has been reviewed and approved by both authors.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Endophthalmitis Vitrectomy Study Group Microbiologic factors and visual outcome in the endophthalmitis vitrectomy study. Am J Ophthalmol 1996;122:830–46 [DOI] [PubMed] [Google Scholar]
- 2.Sachdeva V, Pathengay A, Joseph J, et al. Burkholderia cepacia endophthalmitis: clinico-microbiologic profile and outcomes. Retina 2011;31:1801–5 [DOI] [PubMed] [Google Scholar]
- 3.Anne S, Reisman RE. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Ann Allergy Asthma Immunol 1995;74:167–70 [PubMed] [Google Scholar]
- 4.Craven DE, Moody B, Connolly MG, et al. Pseudobacteremia caused by povidone-iodine solution contaminated with Pseudomonas cepacia. N Engl J Med 1981;305:621–3 [DOI] [PubMed] [Google Scholar]
- 5.Sobel JD, Hashman N, Reinherz G, et al. Nosocomial Pseudomonas cepacia infection associated with chlorhexidine contamination. Am J Med 1982;73:183–6 [DOI] [PubMed] [Google Scholar]
- 6.Englander M, Chen TC, Paschalis EI, et al. Intravitreal injections at the Massachusetts Eye and Ear Infirmary: analysis of treatment indications and postinjection endophthalmitis rates. Br J Ophthalmol 2013;97:460–5 [DOI] [PubMed] [Google Scholar]
- 7.Ng DS, Kwok AK, Chan CW, et al. Intravitreal bevacizumab: safety of multiple doses from a single vial for consecutive patients. Hong Kong Med J 2012;18:488–95 [PubMed] [Google Scholar]
- 8.Lyall DA, Tey A, Foot B, et al. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye (Lond) 2012;26:1517–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moshfeghi AA. Endophthalmitis following intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Semin Ophthalmol 2011;26:139–48 [DOI] [PubMed] [Google Scholar]
- 10.Endophthalmitis Vitrectomy Study Group Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 1995;113:1479–96 [PubMed] [Google Scholar]
- 11.Shimomura H, Matsuura M, Saito S, et al. Unusual interaction of a lipopolysaccharide isolated from Burkholderia cepacia with polymyxin B. Infect Immun 2003;71:5225–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiscella RG, Nguyen TK, Cwik MJ, et al. Aqueous and vitreous penetration of levofloxacin after oral administration. Ophthalmology 1999;106:2286–90 [DOI] [PubMed] [Google Scholar]
- 13.Del Piero E, Pennett M, Leopold I. Pseudomonas cepacia endophthalmitis. Ann Ophthalmol 1985;17:753–6 [PubMed] [Google Scholar]


