Abstract
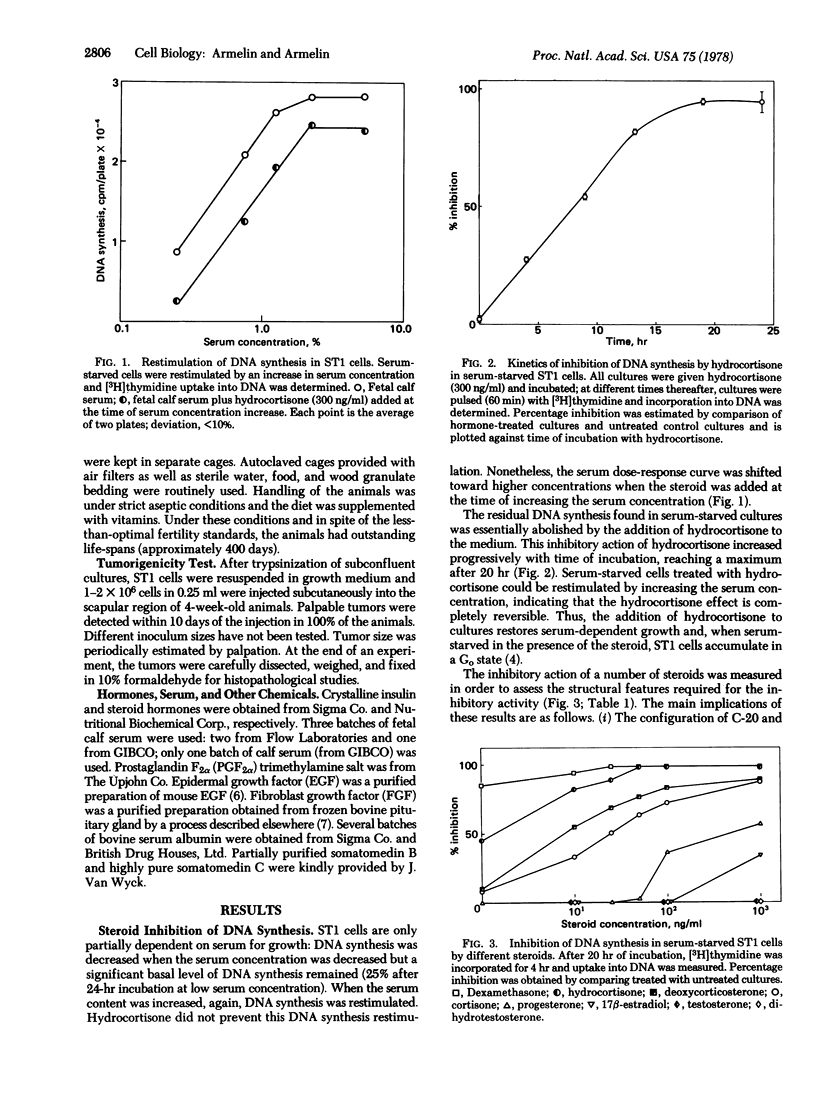
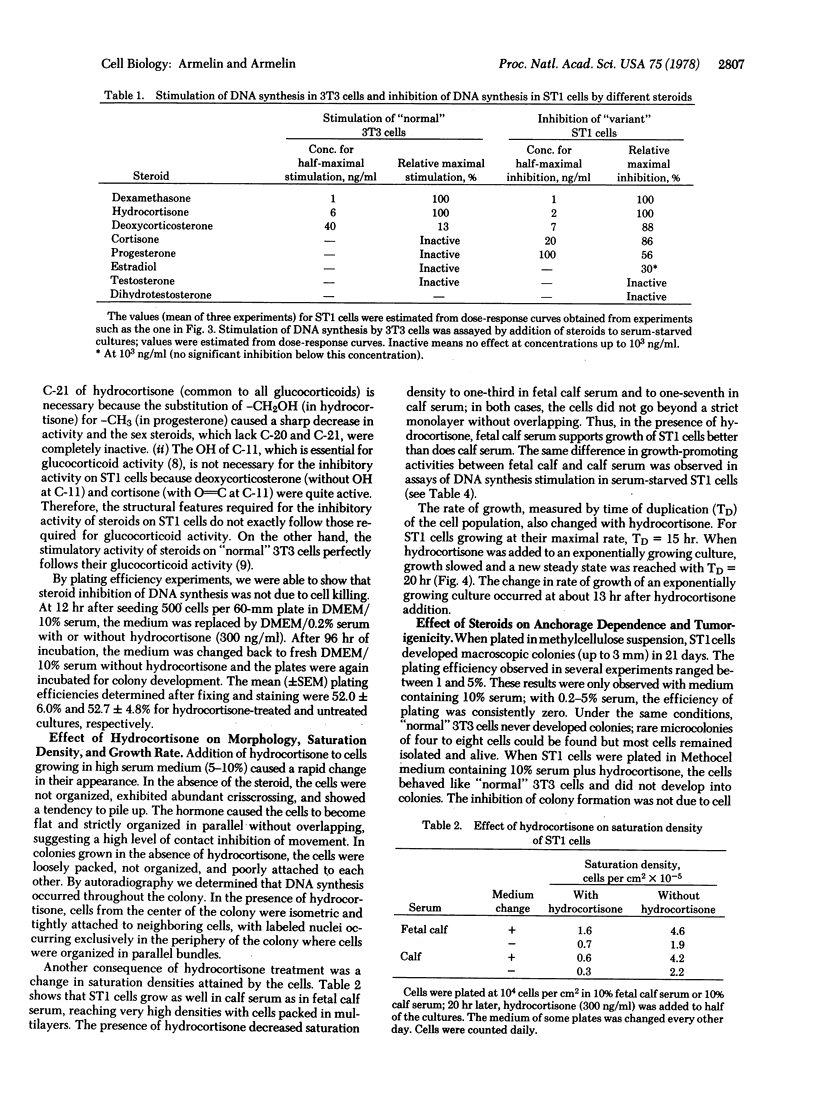
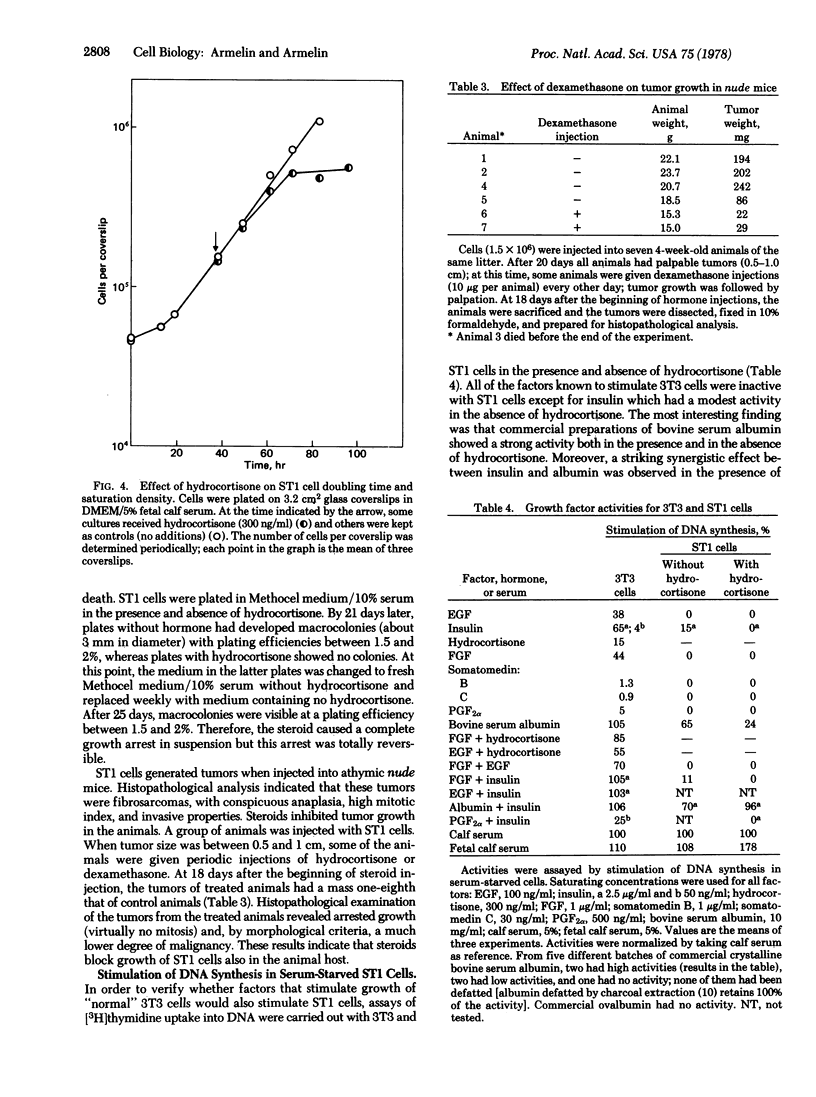
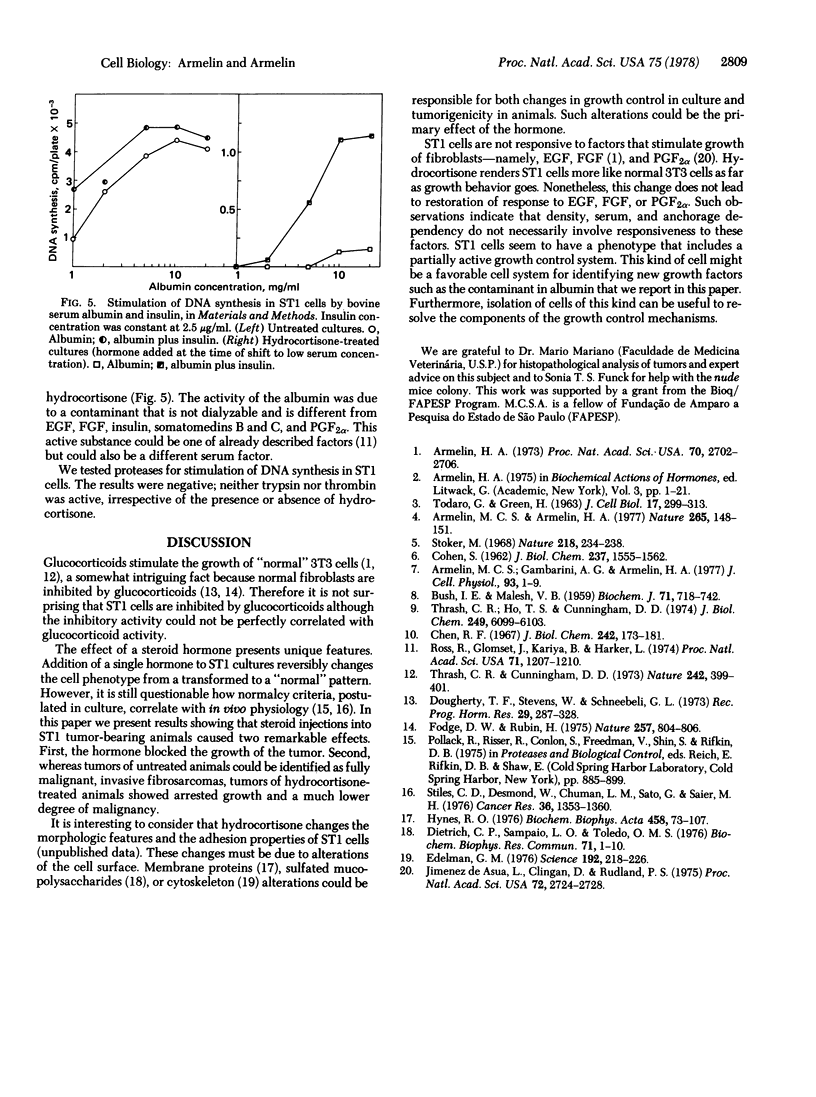
Hydrocortisone at physiological concentrations reversibly inhibits DNA synthesis in ST1 cells (a line of mouse fibroblasts possessing 40 chromosomes and derived from Swiss 3T3 cells). This inhibitory activity is a property of glucocorticoids, but the beta-OH of C-11 of glucocorticoids is not essential for the inhibition. The steroid hormone restores to ST1 cells dependency on serum, density, and anchorage for growth. When injected into nude mice, ST1 cells generated malignant invasive fibrosarcoma. Injections of dexamethasone into tumor-bearing animals blocked tumor growth. The steroid hormone seems to induce a reversible transition between a transformed and a "normal" phenotype. ST1 cells treated or untreated with hydrocotisone are not responsive to fibroblast growth factor, epidermal growth factor, or prostaglandin F2alpha whereas they are responsive to a factor that is a contaminant in bovine serum albumin.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2702–2706. doi: 10.1073/pnas.70.9.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armelin M. C., Armelin H. A. Serum and hormonal regulation of the "resting-proliferative" transition in a variant of 3T3 mouse cells. Nature. 1977 Jan 13;265(5590):149–151. doi: 10.1038/265148a0. [DOI] [PubMed] [Google Scholar]
- BUSH I. E., MAHESH V. B. Metabolism of 11-oxygenated steroids. 2. 2-Methyl steroids. Biochem J. 1959 Apr;71(4):718–742. doi: 10.1042/bj0710718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- De Asua L. J., Clingan D., Rudland P. S. Initiation of cell proliferation in cultured mouse fibroblasts by prostaglandin F2alpha. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2724–2728. doi: 10.1073/pnas.72.7.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. P., Sampaio L. O., Toledo O. M. Characteristic distribution of sulfated mucopolysaccharides in different tissues and in their respective mitochondria. Biochem Biophys Res Commun. 1976 Jul 12;71(1):1–10. doi: 10.1016/0006-291x(76)90241-2. [DOI] [PubMed] [Google Scholar]
- Dougherty T. F., Stevens W., Schneebeli G. L. Functional and morphological alterations produced in target cells by anti-inflammatory steroids. Recent Prog Horm Res. 1973;29:287–328. doi: 10.1016/b978-0-12-571129-6.50011-7. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Differential effects of glucocorticoids on DNA synthesis in normal and virus-transformed chick embryo cells. Nature. 1975 Oct 30;257(5529):804–806. doi: 10.1038/257804a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Desmond W., Chuman L. M., Sato G., Saier M. H. Growth control of heterologous tissue culture cells in the congenitally athymic nude mouse. Cancer Res. 1976 Apr;36(4):1353–1360. [PubMed] [Google Scholar]
- Stoker M. Abortive transformation by polyoma virus. Nature. 1968 Apr 20;218(5138):234–238. doi: 10.1038/218234a0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash C. R., Cunningham D. D. Stimulation of division of density inhibited fibroblasts by glucocorticoids. Nature. 1973 Apr 6;242(5397):399–401. doi: 10.1038/242399a0. [DOI] [PubMed] [Google Scholar]
- Thrash C. R., Ho T. S., Cunningham D. D. Structural features of steroids which initiate proliferation of density-inhibited 3T3 mouse fibroblasts. J Biol Chem. 1974 Oct 10;249(19):6099–6103. [PubMed] [Google Scholar]


