Abstract
Background
Growth differentiation factor 15 (GDF-15) is produced by cardiomyocytes and atherosclerotic lesions under stress conditions. Although higher circulating GDF-15 concentrations are associated with mortality across a spectrum of cardiovascular conditions, the relationship of GDF-15 with atherosclerosis and mortality in the general population remains undefined.
Methods
We measured plasma GDF-15 in 3219 participants of the Dallas Heart Study, a population sample of adults ages 30–65 years (55% women, 49% black). GDF-15 was analyzed in prespecified categories (<1200; 1200–1799; and ≥1800 ng/L) and continuously. End points included prevalent coronary artery calcium (CAC >10 Agatston units), increased CAC (CAC ≥100 Agatston units) by electron beam computed tomography, and mortality through a median 7.3 years of follow-up (120 deaths, 48 cardiovascular deaths).
Results
Increasing GDF-15 associated with older age, black race, hypertension, diabetes, smoking, left ventricular (LV) mass/body surface area, and worse renal function (P < 0.0001 for each). In multivariable models adjusted for traditional risk factors, renal function, and LV mass/body surface area, GDF-15 ≥1800 ng/L was associated with CAC >10 (odds ratio 2.1; 95% CI 1.2–3.7; P = 0.01), CAC ≥100 (odds ratio 2.6; 95% CI 1.4–4.9; P = 0.002), all-cause mortality (hazard ratio 3.5; 95% CI 2.1–5.9, P < 0.0001), and cardiovascular mortality (hazard ratio 2.5; 95% CI 1.1–5.8, P = 0.03). Adding log GDF-15 to fully adjusted models modestly improved the c statistic (P = 0.025), the integrated discrimination index (0.028; P < 0.0001) and the category-less net reclassification index (0.42; P = 0.002). These findings remained significant with further adjustment for high-sensitivity C-reactive protein, N-terminal pro–B-type natriuretic peptide, and cardiac troponin T.
Conclusions
GDF-15 is independently associated with subclinical coronary atherosclerosis and mortality, and its potential role for risk stratification in the general population merits further evaluation.
Growth differentiation factor 15 (GDF-15),5 a member of the transforming growth factor superfamily, is produced and secreted by cardiomyocytes, activated macrophages (1, 2), endothelial cells (3), vascular smooth muscle cells (4), and adipocytes (5). In ex vivo and in vivo murine models, as well as human cardiomyocytes, GDF-15 is upregulated and actively secreted from areas of myocardial infarction and ischemia and, via presumed paracrine effects, reduces infarct size, apoptosis, and hypertrophy (2, 6).
In humans, circulating GDF-15 robustly associates with mortality across a spectrum of cardiovascular disease states including chest pain, acute coronary syndromes, stable coronary heart disease (CHD), and heart failure (7–12). Given the consistency of these associations across multiple cardiovascular disease phenotypes, GDF-15 has emerged as a promising novel biomarker for risk assessment (13, 14). To date, however, few data are available evaluating the prognostic utility of GDF-15 in the general population; its potential utility for cardiovascular risk assessment among low-risk individuals without established cardiovascular disease remains unclear.
We assessed associations between plasma concentrations of GDF-15 and subclinical coronary atherosclerosis and mortality in the Dallas Heart Study (DHS), a probability-based population sample of ethnically diverse adults.
Methods
Study Population
The Dallas Heart Study is a probability-based sample of 6101 Dallas County residents ages 18–65 years, with intentional oversampling of self-identified African Americans (15). Data collection occurred during 3 visits from 2000 to 2002. Visit 1 (n = 6101) entailed in-home collection of demographic and survey information along with blood pressure and anthropometric measurements for all participants. A subset of participants from visit 1 between the ages of 30 and 65 years received a second in-home visit (n = 3398) for the collection of blood and urine samples. A final study visit was conducted at the University of Texas Southwestern Medical Center at Dallas among participants completing visit 2 (n = 2971), where detailed cardiac and clinical phenotyping was performed. Demographics, medical history, blood pressure, and body composition were similar between participants completing visits 1 and 2, and standard laboratory values did not differ significantly between those completing visits 2 and 3 (15). The present study is based on the 3291 participants from the DHS ages 30–65 years with GDF-15 measurements, of whom 2564 underwent coronary artery calcium scans and 2596 underwent cardiac MRI. This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and was conducted in accordance with institutional guidelines. All participants provided written informed consent.
Study Definitions
Demographic information, anthropometric measurements, and other variable definitions have been described in detail (15). Hypercholesterolemia was defined as a calculated LDL cholesterol ≥160 mg/dL (≥4.14 mmol/L) on a fasting sample, direct LDL cholesterol ≥160 mg/dL (≥4.14 mmol/L) on a nonfasting sample, total cholesterol ≥240 mg/dL (≥6.21 mmol/L), or use of statin medication. Low HDL cholesterol was defined as HDL cholesterol <40 mg/dL (<1.03 mmol/L) in men and <50 mg/dL (<1.29 mmol/L) in women. Hypertension was defined as average systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication. Diabetes was defined as a fasting glucose level ≥126 mg/dL (>7.0 mmol/L), nonfasting glucose >200 mg/dL (>11.1 mmol/L), or self-reported diabetes coupled with the use of any glucose-lowering medication. History of cardiovascular disease (CVD) was defined as a self-reported history of myocardial infarction (MI), congestive heart failure (CHF), or stroke or evidence of coronary revascularization on electron beam computed tomography (EBCT). We calculated the estimated glomerular filtration rate (eGFR) using the abbreviated Modification of Diet and Renal Disease calculation, where eGFR [mL · min−1 · (1.73 m2)−1] = 186 × (Scr in mg/dL)1.154 × (age in years) −0.203 × 0.742 (if female) × 1.21 (if African American), where Scr is serum creatinine concentration. eGFR was categorized into 5 clinically driven categories as defined by the National Kidney Foundation clinical practice guidelines: stage I (normal) [eGFR ≥90 mL · min−1 · (1.73 m2)−1], stage II (reduced GFR) [60 ≤ eGFR <90 mL · min−1 · (1.73 m2)−1], stage III [30 ≤ eGFR <60 mL · min−1 · (1.73 m2)−1], stage IV [15 ≤ eGFR <30 mL · min−1 · (1.73 m2)−1], and stage V [eGFR <15mL · min−1 · (1.73 m2)−1 or on dialysis].
Imaging and Mortality End Points
We measured coronary artery calcium (CAC) twice by EBCT and averaged the results, with prevalent CAC defined by an average score of >10 Agatston units, a data-derived threshold determined to maximize signal-to-noise ratio, as described (16).
The MRI protocol to assess cardiac structure and function has been described (17). We obtained short-axis breath-hold, electrocardiographic-gated cine 1.5 Tesla MR images from the apex to the base of the left ventricle and used MASS (Medis Medical Imaging Systems) software to analyze the data. Endocardial and epicardial borders were traced manually, allowing calculation of ventricular volumes. Left ventricular (LV) mass, wall thickness, and ejection fraction (LVEF) were calculated as described (17). LV mass was indexed to body surface area.
We ascertained all-cause and cardiovascular mortality using National Death Index data through December 31, 2008 [median follow-up time 7.3 (IQR 7.0–7.8) years]. Cardiovascular death was defined using International Classification of Diseases, Revision 10 (ICD-10) codes I00–I99 (18).
Measurement of GDF-15 and Other Biomarkers
Venous blood was collected in standard blood collection tubes containing citrate EDTA, and samples were maintained at 4 °C for ≤4 h and then centrifuged (1430gfor 15 min) at 4 °C. Plasma was then removed and frozen at −80 °C until assays were performed. We measured GDF-15 from thawed frozen plasma at Alere San Diego, Inc., using a proprietary sandwich platform (minimum detection limit 10 ng/L, maximum cutoff 7500 ng/L, within-assay CV 6%, interassay CV 9%). Assays were performed by individuals blinded to all clinical data. Samples had been thawed once for aliquoting before biomarker measurement. As previously described, we measured high-sensitivity C-reactive protein (hsCRP) (19) and N-terminal pro–B-type natriuretic peptide (NT-proBNP) (20) using standard commercial assays (Roche Diagnostics), and cardiac troponin T (cTnT) with a new highly sensitive assay (Elecsys-2010 Troponin T hs STAT, Roche Diagnostics) (21).
Statistical Analysis
All analyses were based on the 3291 participants with available GDF-15 measurements. We analyzed GDF-15 primarily as a categorical variable based on prior published cut points: <1200 ng/L; 1200–1799 ng/L, and ≥1800 ng/L (10, 22). By way of sensitivity analyses, we also analyzed GDF-15 as a log-transformed continuous covariate in all models. We used age-adjusted Spearman correlations to assess univariable associations between GDF-15 and continuous cardiovascular risk factors and biomarkers. All models assessing associations between GDF-15 and CAC and mortality end points were adjusted for age, sex, race, hypertension, diabetes, current smoking, hypercholesterolemia (including statin use), low HDL cholesterol, body mass index (BMI), chronic kidney disease (CKD) stages, LV mass/body surface area, and history of CVD. Sensitivity analyses were conducted for all models excluding participants with a history of CVD. Serial adjustments were made for hsCRP ≥75th percentile, NT-proBNP ≥75th percentile, and cTnT ≥75th percentile (all subjects with detectable cTnT with the highly sensitive assay). For models using log GDF-15 as a continuous variable, we also analyzed eGFR, log hsCRP, log NT-proBNP, and quintiles of cTnT (21) as continuous variables. We performed univariable and multivariable logistic regression analyses to assess associations of GDF-15 with prevalent CAC (score >10) and increased CAC (score ≥100) (23). We used multivariable Cox proportional hazards models to assess associations of GDF-15 with all-cause death. Proportional hazards assumptions were met for all models.
We analyzed the additional contribution of log GDF-15 beyond traditional risk factors in predicting all-cause death using multiple metrics of biomarker performance, including discrimination (c statistic), calibration (Hosmer–Lemeshow), and reclassification [integrated discrimination index (IDI) and net reclassification index (NRI)]. All models included the following risk factors: age, sex, race, hypertension, diabetes, current smoking, hypercholesterolemia, low HDL cholesterol, BMI, eGFR, LV mass/body surface area, and history of CVD. We also assessed incremental performance of log GDF-15 after serial inclusion of log hsCRP, log NT-proBNP, and quintiles of cTnT. We calculated time-dependent c statistics from Cox proportional hazards models using the Chambless c statistic (24, 25), which directly incorporates survival function estimates. Improved discrimination is reflected in higher c statistics. The IDI is a continuous measure of reclassification and reflects the difference in discrimination slopes between 2 models, with positive values reflecting improved discrimination. Time-dependent IDI was derived from Cox proportional hazards models as described by Pencina et al. (26). Calibration of time-dependent models was assessed using a modified Hosmer–Lemeshow χ2 statistic (27), with higher P values indicating better calibrated models. Because there were no established thresholds of predicting all-cause death, we calculated a category-less NRI from time-dependent models (28). In addition, we used the Wald χ2 values for each covariate derived from fully adjusted Cox proportional hazards models for all-cause mortality to assess the contribution of GDF-15 and other markers to model performance. All statistical analyses were performed using SAS Version 9.2. For all statistical testing, 2-sided P values were reported, and a P value <0.05 was considered statistically significant without correction for multiple comparisons.
Results
The median [25th, 75th percentile] plasma concentration of GDF-15 in all participants was 670 ng/L [490, 930], and in those without prevalent CVD, was 650 ng/L [479, 889]. Previously reported GDF-15 cut points (10, 22) of 1200 ng/L corresponded to the 89th percentile and 1800 ng/L the 96.3th percentile in participants without CVD. In unadjusted analyses, increasing categories of GDF-15 associated with increasing age, black race, hypertension, diabetes, smoking, and LV mass/body surface area and were inversely associated with renal function and LDL cholesterol (Table 1). GDF-15 concentrations did not associate with sex, BMI, or other anthropometric measures. Age was the most strongly correlated continuous variable with increasing GDF-15 (ρ = 0.42, P < 0.0001), followed by NT-proBNP, cTnT, LV mass, and hsCRP (P < 0.0001 for all) (Table 2). LDL cholesterol, total cholesterol, and estimated GFR were inversely correlated with GDF-15 (P < 0.0001 for all) (Table 2).
Table 1.
Cardiovascular risk factors and biomarkers by GDF-15 categories: general population.a
| GDF-15 concentration | ||||
|---|---|---|---|---|
| Variable | <1200 ng/L | 1200–1799 ng/L | ≥1800 ng/L | P trend |
| n | 2846 | 295 | 150 | |
| Age | 42 (35, 50) | 52 (46, 57) | 52 (45, 58) | <0.0001 |
| Men, % | 44 | 44 | 53 | 0.06 |
| Black, % | 50 | 60 | 63 | 0.0002 |
| White, % | 30 | 28 | 17 | 0.0002 |
| BMI, kg/m2 | 29.4 (25.6, 34.4) | 28.2 (24.3, 34.8) | 30.5 (23.9, 36.2) | 0.21 |
| Hypertension, % | 30 | 53 | 63 | <0.0001 |
| Diabetes, % | 9 | 21 | 34 | <0.0001 |
| Current smoking, % | 27 | 45 | 40 | <0.0001 |
| History of CVD, % | 15 | 33 | 33 | <0.0001 |
| Total cholesterol, mg/dL | 177 (154, 203) | 175 (151, 201) | 166 (143, 191) | 0.002 |
| LDL cholesterol, mg/dL | 105 (85, 127) | 98 (73, 124) | 84 (66, 112) | <0.0001 |
| HDL cholesterol, mg/dL | 47 (40, 57) | 47 (39, 61) | 48 (40, 59) | 0.52 |
| Triglycerides, mg/dL | 94 (67, 143) | 104 (73, 158) | 113 (81, 168) | <0.0001 |
| Non-HDL cholesterol, mg/dL | 128 (104, 154) | 121 (94, 153) | 113 (92, 142) | <0.0001 |
| Systolic blood pressure, mm HG | 121 (111, 133) | 128 (116, 143) | 129 (116, 148) | <0.0001 |
| LV mass/body surface area, g/m2 | 79.8 (69.8, 92.3) | 83.2 (71.0, 100.4) | 86.8 (74.0, 98.4) | <0.0001 |
| LVEF, % | 73 (68, 77) | 73 (67, 78) | 74 (67, 79) | 0.55 |
| eGFR, mL· min−1 · (1.73 m2)−1 | 98.6 (85.9, 113.4) | 89.4 (76.9, 109) | 84.6 (65.4, 109.8) | <0.0001 |
| hsCRP, g/L | 2.7 (1.1, 6.7) | 4.1 (1.8, 9.4) | 4.4 (1.4, 9.9) | <0.0001 |
| NT-proBNP, ng/L | 26.7 (12.2, 54.6) | 48.8 (25.8, 102.5) | 60.9 (28.2, 218) | <0.0001 |
| cTnT, % detectable | 24 | 43 | 61 | <0.0001 |
Data are median (IQR) for continuous variables or % for categorical variables. CVD, self-reported history of MI, CHF, or stroke or evidence of median sternotomy or coronary stent on EBCT. For conversion of cholesterol and triglycerides concentrations to mmol/L, multiply by 0.2586 and 0.01129, respectively.
Table 2.
Age-adjusted with Spearman correlations GDF-15.
| General population (n = 3291) | Without CVD (n = 2726)a | |||
|---|---|---|---|---|
| Spearman ρ | P value | Spearman ρ | P value | |
| NT-proBNP | 0.14 | <0.0001 | 0.10 | <0.0001 |
| cTnT | 0.14 | <0.0001 | 0.10 | <0.0001 |
| LDL cholesterol | −0.11 | <0.0001 | −0.11 | <0.0001 |
| eGFR | −0.10 | <0.0001 | −0.07 | 0.0006 |
| LV mass/body surface area | 0.09 | <0.0001 | 0.05 | 0.001 |
| Total cholesterol | −0.08 | <0.0001 | −0.07 | 0.0002 |
| hsCRP | 0.07 | <0.0001 | 0.07 | 0.0002 |
| Systolic blood pressure | 0.05 | 0.01 | 0.06 | 0.006 |
| Triglyceride | 0.03 | 0.08 | 0.03 | 0.13 |
| BMI | −0.02 | 0.27 | −0.02 | 0.23 |
| HDL cholesterol | −0.01 | 0.61 | 0.01 | 0.68 |
CVD, self-reported history of MI, CHF, or stroke or evidence of median sternotomy or coronary stent on EBCT.
Association of GDF-15 with Prevalent CAC
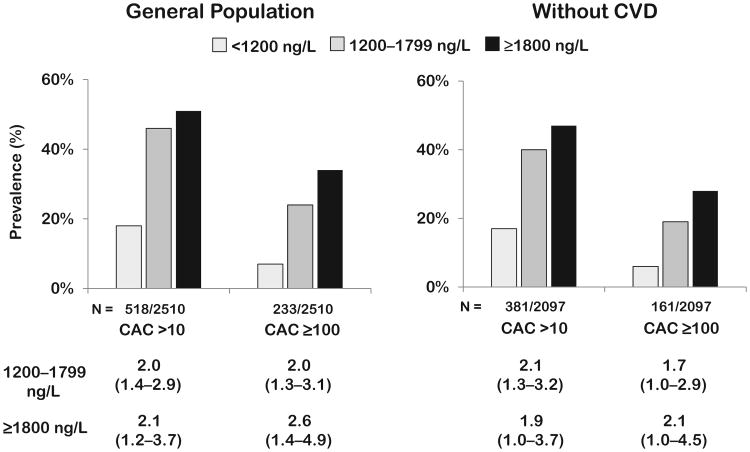
In unadjusted analyses, both prevalent CAC and CAC ≥100 increased significantly across GDF-15 categories (Ptrend <0.0001 for both) (Fig. 1). These associations persisted in fully adjusted analyses (odds ratio for GDF ≥1800 ng/L: CAC ≥10, 2.1, 95% CI 1.2–3.7, P = 0.01; CAC ≥100, 2.6, 95% CI 1.4–4.9, P = 0.002). Analyzing GDF-15 as a continuous variable confirmed significant associations with prevalent CAC and CAC ≥100 (see Supplemental Fig. 1, which accompanies the online version of this article at http://www.clinchem.org/content/vol58/issue1).
Fig. 1. Prevalent coronary calcium by GDF-15 groups.
Adjusted odds ratios calculated for GDF-15 groups compared with GDF-15 <1200 ng/L, from logistic regression models for CAC >10 and CAC ≥ 100 in the general population and in those without cardiovascular disease, adjusting for age, sex, hypertension, diabetes, smoking, hypercholesterolemia, low HDL cholesterol, BMI, black or Hispanic race, eGFR, and LV mass/body surface area. Model for general population additionally adjusted for history of cardiovascular disease. CVD, self-reported history of MI, CHF, or stroke or evidence of median sternotomy or coronary stent on EBCT.
Association of GDF-15 with Mortality
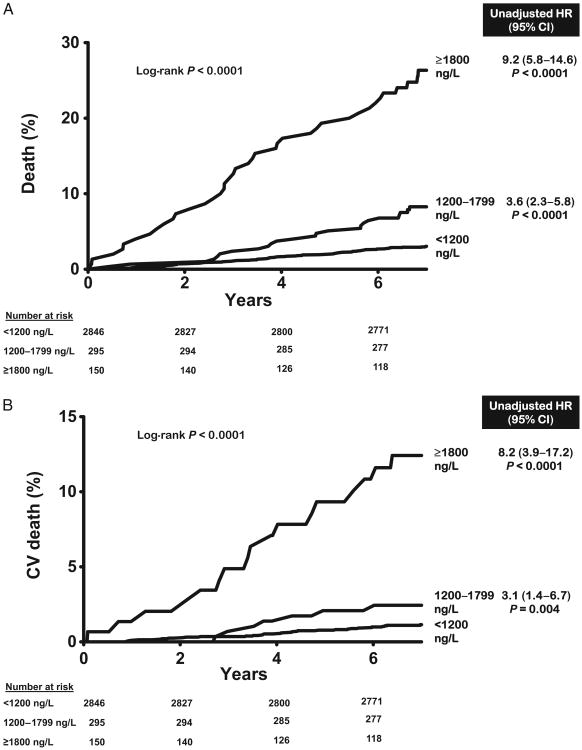
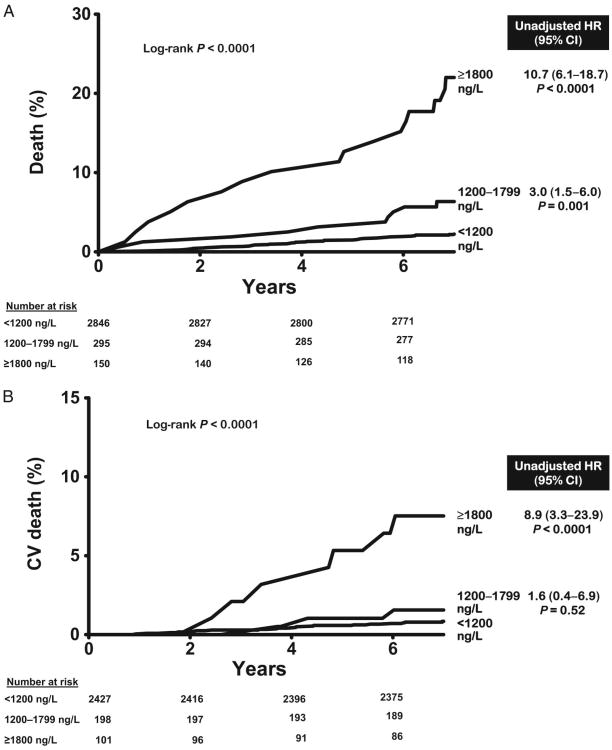
Over a median follow-up of 7.3 years [interquartile range (IQR) 7.0–7.8], there were 120 total deaths and 48 cardiovascular deaths. All-cause mortality was 3.2% in the lowest GDF-15 category (<1200 ng/L, n = 2846), 9.8% in the intermediate category (1200–1799 ng/L, n = 295), and 26.0% in the highest category (≥1800 ng/L, n = 150) (log-rank P < 0.0001) (Fig. 2A). Similar associations were seen for cardiovascular mortality (Fig. 2B). In Cox proportional hazards models adjusting for age, sex, race, hypertension, diabetes, current smoking, hypercholesterolemia, low HDL cholesterol, BMI, CKD stage, LV mass/body surface area, and history of CVD (base model), participants with GDF-15 concentrations ≥1800 ng/L were at increased risk for all-cause [hazard ratio (HR) 3.5, 95% CI 2.1–5.9, P < 0.0001] and cardiovascular (HR 2.5, 95% CI 1.1 –5.8, P = 0.03) death compared to those with <1200 ng/L (Fig. 2). Further adjustment for hsCRP, NT-proBNP, and cTnT only modestly attenuated the associations with all-cause mortality (HR 3.0, 95% CI 1.7 –5.1, P < 0.0001) and cardiovascular death (HR 1.9, 95% CI 0.8–4.8, P = 0.1). When analyzed as a continuous variable, log GDF-15 was associated independently with all-cause (HR per 1 log unit increase 2.4, 95% CI 1.7–3.4, P < 0.0001) and cardiovascular (HR 1.8, 95%CI 1.1–3.2, P = 0.03) death (see online Supplemental Fig. 1). Use of continuous forms of risk factors, including total cholesterol, HDL cholesterol, systolic blood pressure, and eGFR, did not significantly alter the associations. Exclusion of participants with a history of CVD yielded qualitatively similar results (Fig. 3 and online Supplemental Fig. 1).
Fig. 2. GDF-15 groups and mortality in the general population.
(A), All-cause mortality. (B), Cardiovascular mortality. Unadjusted hazard ratios calculated for GDF-15 groups compared with GDF-15 <1200 ng/L, from Cox proportional hazards models for all-cause death (120/2754) and cardiovascular death (48/2754) in the general population.
Fig. 3. GDF-15 groups and mortality in those without cardiovascular disease.
(A), All-cause mortality. (B), Cardiovascular mortality. Unadjusted hazard ratios calculated for GDF-15 groups compared with GDF-15 <1200 ng/L, from Cox proportional hazards models for all-cause death (77/2288) and cardiovascular death (26/2288) in those without cardiovascular disease.
For all-cause death, addition of log GDF-15 to the base model consisting of the covariates listed above significantly improved the c statistic (from 0.822 to 0.839; P = 0.025), the time-dependent IDI (0.028; P < 0.0001), and the category-less NRI (0.42; P = 0.002) (Table 3). Moreover, when added to the base model, log GDF-15 had the highest Wald χ2 value of any of the variables (see online Supplemental Fig. 2). When log GDF-15 was added to a model that contained hsCRP, NT-proBNP, and cTnT in addition to the base model, log GDF-15 did not significantly improve the c statistic but did improve the IDI (0.01; P = 0.01) and NRI (0.29; P = 0.01) (Table 3). Model calibration for all-cause death improved when log GDF-15 was added to a model containing hsCRP, NT-proBNP, and cTnT (Hosmer–Lemeshow P value increase from 0.18 to 0.50).
Table 3.
Incremental performance of log GDF-15 for all-cause death from time-dependent models.a
| Chambless c statistic | IDI | Relative IDI | NRI | |
|---|---|---|---|---|
| Model 1 | ||||
| Risk factors (RF) | 0.822 (0.786–0.858) | |||
| Model 2 | ||||
| RF + GDF-15 | 0.839 (0.805–0.873)b | 0.028 (0.015–0.041)b | 0.26 | 0.42 (0.15–0.63)b |
| Model 3 | ||||
| RF + hsCRP + NT-proBNP + cTnT | 0.834 (0.800–0.867)c | 0.040 (0.019–0.061)c | 0.37 | 0.25 (0.03–0.43)c |
| Model 4 | ||||
| Model 3 + GDF-15 | 0.842 (0.810–0.874)d | 0.010 (0.002–0.018)d | 0.07 | 0.29 (0.11–0.47)d |
Data are mean (95% CI). All markers (GDF-15, hs-CRP, NT-proBNP, and cTnT) were log-transformed. Chambless c-statistic, time-dependent IDI, and category-less NRI derived from Cox proportional hazards models for all-cause death. Risk factors included age, sex, hypertension, diabetes, smoking, hypercholesterolemia, low HDL-C, BMI, black or Hispanic race, eGFR, LV mass/BSA, and history of CVD.
Model 2 vs model 1: P = 0.025 for Chambless c statistic; P <0.0001 for IDI; P = 0.002 for NRI.
Model 3 vs model 1: P = 0.08 for Chambless c statistic; P = 0.0002 for IDI; P = 0.03 for NRI.
Model 4 vs Model 3: P = 0.12 for Chambless c-statistic; P = 0.01 for IDI; P = 0.01 for NRI.
Discussion
In a multiethnic cohort of middle-aged adults in the general population, we found that increasing plasma concentrations of GDF-15 were associated with a number of CHD risk factors, prevalent subclinical coronary atherosclerosis, and subsequent all-cause and cardiovascular death. Prior reports have established associations of GDF-15 with mortality in older cohorts free of CHD (29), as well as patients with stable CHD (30), acute coronary syndromes (7, 9, 10, 30), and CHF (8). In this report, we extend the associations between GDF-15 and cardiovascular risk, subclinical atherosclerosis, and cardiovascular and all-cause mortality risk to a younger, multiethnic cohort that is representative of the general population.
GDF-15 Regulation
GDF-15 is a member of the transforming growth factor superfamily and is secreted from activated macrophages by stimulation from proinflammatory cytokines (1), as well as from human endothelial cells (3), human vascular smooth muscle cells (4), and human adipocytes (5). It is therefore plausible that circulating concentrations would be increased in individuals with atherosclerosis, a chronic inflammatory condition involving macrophage accumulation in lipid-laden arterial plaques (31). In support of this hypothesis, GDF-15 expression increases in human atherosclerotic carotid artery specimens in response to oxidized LDL (32). Whether GDF-15 contributes directly to atherosclerosis development has not been established. The absence of induction of GDF-15 in macrophages after direct stimulation by lipopolysaccharide suggests a possible counter-regulatory effect of GDF-15 rather than a primary effect on macrophage activation (1). Infusion of re-combinant GDF-15 into infarcted myocardium suppresses the inflammatory response, also suggesting a counterregulatory cytokine role (33). GDF-15 has also been found to be secreted from myocardial tissue in response to ischemia and reperfusion in murine models and is expressed in infarcted human myocardium (2, 6), further supporting its role as a potential cardiovascular biomarker.
GDF-15 and Cardiovascular Risk Factors
Prior reports of associations between circulating GDF-15 and cardiovascular disease have largely been restricted to elderly individuals (22) or those with chronic or acute cardiovascular disease (7–10, 30), and all have been performed in predominantly male Europeans with normal orborderline BMI. One exception is a small nested case-control study from the Women's Health Study (34). In those previous studies, increasing GDF-15 has been consistently associated with increasing age, diabetes, renal dysfunction, and inflammatory markers such as CRP, consistent with our findings in a younger population free of cardiovascular disease. Reported associations between GDF-15 and BMI, smoking, hypertension, and NT-proBNP have been less consistent. In our younger multiethnic study cohort of mostly overweight to obese individuals, 55% of whom were women, increasing GDF-15 was significantly correlated with black race, smoking, hypertension, and increasing NT-proBNP concentrations, but there were no associations with BMI and no differences by sex.
GDF-15, CVD, and Mortality
In a nested case-control analysis within the Women's Health Study, GDF-15 concentrations measured by a murine monoclonal antibody were associated with nonfatal MI and stroke in healthy women (median age 60), with levels above the 90th percentile associated with a 2.7-fold increased risk after adjustment for other risk factors (34). In our population-based study of younger men and women, increasing GDF-15 concentrations were associated with prevalent CAC and increased CAC (≥100). Our findings extend the only prior reported associations between GDF-15 and atherosclerosis in humans, which demonstrated an association between GDF-15 and extent of coronary disease in patients presenting with acute coronary syndromes (35) and between GDF-15 and carotid plaque in elderly individuals (36). Interestingly, although there was only a weak age-adjusted correlation between LDL cholesterol and GDF-15, the correlation was inverse despite increased CAC and other CVD risk factors with increasing GDF-15 concentrations, a finding also seen by another study in older community dwellers (29).
GDF-15 has been evaluated previously in several high-risk populations with ischemic heart disease, including patients with ST-elevation MI (7, 9), non–ST-elevation MI (10), and stable CHD (30). In these patient populations, increasing concentrations of GDF-15 were associated with total and cardiovascular mortality but inconsistently with nonfatal MI. In these first studies, a threshold of 1200 ng/L was derived as the upper limit of normal (90th percentile) in healthy elderly European men and women, and also represented the lower tertile boundary in a trial of patients presenting with acute coronary syndromes (10). A threshold of 1800 ng/L represented the upper tertile boundary in this same high-risk population and was associated with increased mortality risk (10). ROC curve analyses within the same cohorts confirmed the rounded value of 1800 ng/L as the optimal concentration for prediction of 1-year mortality in patients presenting with acute coronary syndromes, and subsequent studies have validated the 1800 ng/L threshold for increased mortality risk (35). These associations with mortality have been extended to a stable CHF population (8) and patients presenting with chest pain without MI (11) and have been replicated by an independent group in a European post-MI sample (9) as well as in older community dwellers (29). Furthermore, these consistent associations with mortality are independent of levels of myocardial necrosis markers and natriuretic peptides. In our much younger population-based study cohort, we report similar associations between increasing GDF-15 concentrations and all-cause and cardiovascular mortality, with up to a 3-fold increased risk of all-cause mortality in those with baseline GDF-15 ≥1800 ng/L compared to those with < 1200 ng/L after adjusting for traditional risk factors as well as hsCRP, NT-proBNP, and cTnT. These associations with all-cause mortality persisted when participants with a history of CVD were excluded.
Most prior human studies of GDF-15 and CVD have been performed by a single investigative group using an assay developed in their laboratory (22). It is thus encouraging that our independent findings using a different assay are similarly robust. The median concentration of GDF-15 in the DHS (650 ng/L) was similar to the range previously reported for healthy participants <60 years old (circa 675 ng/L) using another assay (22). Similar to observations in high-risk populations, most of the increased mortality risk in the DHS cohort occurred in the group with baseline GDF-15 ≥1800 ng/L, with a 26% incidence of death over a median follow-up of 7.3 years, a risk 8-fold higher than among those with GDF-15 <1200 ng/L, and remarkable given the overall low risk of the cohort.
Despite consistent associations between increased GDF-15 and mortality risk across the spectrum of baseline cardiovascular risk, the clinical utility of GDF-15 measurement to further refine cardiovascular risk and dictate treatment strategies has not been well studied (14). In the AtheroGene registry, addition of GDF-15 to risk factors improved the c statistic, a measure of discrimination, for CHD mortality in patients with stable angina (CHD) but not in patients with acute coronary syndromes (30). In patients presenting with chest pain, the addition of GDF-15 to the Global Registry of Acute Coronary Events (GRACE) score, a validated risk assessment tool for mortality in acute coronary syndromes, yielded significant improvement in the c statistic as well as the IDI (11). In our population-based cohort, addition of GDF-15 to standard CVD risk factors resulted in modest but significant improvements in the c statistic (discrimination) as well as reclassification, as measured by the IDI and NRI for all-cause death. Compared to the Rancho Bernardo study of older participants (mean age 70) (29), the category-less NRI for log GDF-15 in our younger population (mean age 44) was higher (0.42 vs 0.30) (28), suggesting that the clinical utility of GDF-15 may be greater in younger populations.
The consistent improvement in risk prediction for all-cause mortality with the addition of GDF-15 was maintained despite adjustment for LV mass as measured by cardiac MRI, further supporting that GDF-15 adds prognostic information independent of not only standard cardiovascular risk factors but also structural heart disease. This is the first study to report a significant association between GDF-15 concentrations and subclinical coronary atherosclerosis (CAC) as well as the first to report associations with mortality independent of a newer, highly-sensitive cTnT assay that detected circulating troponin T in almost all individuals with existing CHD (37) and in 25% of our population-based sample (21). When the findings from multiple risk prediction metrics are considered together, we conclude that GDF-15 provides modest but significant clinical utility for predicting all-cause death in our population-based sample. Measuring GDF-15 in cohorts with longer follow-up periods assessing clinically relevant nonfatal cardiovascular events and mortality will allow for better assessments of the clinical utility of using GDF-15 concentrations in improving cardiovascular risk prediction in the general population.
Limitations
The DHS is a relatively young, population-based cohort, and the estimates for mortality in the present study are based on a relatively small number of total deaths and cardiovascular deaths, with resulting limited statistical power. Cardiovascular deaths were classified using ICD-10 codes and are prone to misclassification. Owing to study sampling design, black participants comprised 50% of the cohort, 60% of the group with GDF-15 ≥1800 ng/L, and 75% of the total deaths. Therefore, caution should be used in extrapolating these findings to dissimilar populations.
Conclusion
In a multiethnic probability-based cohort representative of the general population, circulating GDF-15 was independently associated with subclinical coronary atherosclerosis and all-cause and cardiovascular mortality. The potential utility of GDF-15 measurement for population screening of cardiovascular risk warrants further evaluation.
Supplementary Material
Acknowledgments
Research Funding: Grant support for the Dallas Heart Study was provided by the Donald W. Reynolds Foundation at University of Texas Southwestern Medical Center and the US Public Health Service, General Clinical Research Center grant M01-RR00633 from NIH/National Center for Research Resources/Clinical Research. Assay measurements for GDF-15 were provided by Alere San Diego, Inc., under contract agreement between Alere, Inc., and University of Texas Southwestern Medical Center. A. Rohatgi, North and Central Texas Clinical and Translational Science Initiative (NIH UL1-RR024982); J.D. Berry, National Heart, Lung, and Blood Institute and American Heart Association; D.K. McGuire, Alere, Inc.; J.A. de Lemos, Roche Diagnostics and Alere, Inc.
Role of Sponsor: The funding organizations played a direct role in the final approval of manuscript.
Footnotes
Nonstandard abbreviations: GDF-15, growth differentiation factor 15; CHD, coronary heart disease; DHS, Dallas Heart Study; CVD, cardiovascular disease; MI, myocardial infarction; CHF, congestive heart failure; EBCT, electron beam computed tomography; eGFR, estimated glomerular filtration rate; CAC, coronary artery calcium; LV, left ventricular; LVEF, LV ejection fraction; ICD-10, International Classification of Diseases, Revision 10; hsCRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro–B-type natriuretic peptide; cTnT, cardiac troponin T; CKD, chronic kidney disease; IDI, integrated discrimination index; NRI, net reclassification index; IQR, interquartile ran≥ HR, hazard ratio; GRACE, Global Registry of Acute Coronary Events.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors' Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: D.K. McGuire, Tethys Bioscience Inc.; J.A. de Lemos, Tethys Biomedical and Johnson & Johnson.
Stock Ownership: None declared.
Honoraria: J.D. Berry, Merck.
Expert Testimony: None declared.
References
- 1.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–9. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kempf T, Eden M, Strelau J, Naguib M, Willen-bockel C, Tongers J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–60. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 3.Secchiero P, Corallini F, Gonelli A, Dell'Eva R, Vitale M, Capitani S, et al. Antiangiogenic activity of the MDM2 antagonist nutlin-3. Circ Res. 2007;100:61–9. doi: 10.1161/01.RES.0000253975.76198.ff. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez B, Lopez S, Pacheco YM, Villar J, Muriana FJ, Hoheisel JD, et al. Influence of postprandial triglyceride-rich lipoproteins on lipid-mediated gene expression in smooth muscle cells of the human coronary artery. Cardiovasc Res. 2008;79:294–303. doi: 10.1093/cvr/cvn082. [DOI] [PubMed] [Google Scholar]
- 5.Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009;150:1688–96. doi: 10.1210/en.2008-0952. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342–50. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 7.Kempf T, Bjorklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, et al. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J. 2007;28:2858–65. doi: 10.1093/eurheartj/ehm465. [DOI] [PubMed] [Google Scholar]
- 8.Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–60. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 9.Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, et al. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J. 2009;30:1057–65. doi: 10.1093/eurheartj/ehn600. [DOI] [PubMed] [Google Scholar]
- 10.Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–71. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 11.Eggers KM, Kempf T, Venge P, Wallentin L, Wollert KC, Lindahl B. Improving long-term risk prediction in patients with acute chest pain: the Global Registry of Acute Coronary Events (GRACE) risk score is enhanced by selected non-necrosis biomarkers. Am Heart J. 2010;160:88–94. doi: 10.1016/j.ahj.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, et al. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan heart failure trial. Circulation. 2010;122:1387–95. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 13.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115:949–52. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
- 14.Rohatgi A, de Lemos JA. The report card on growth differentiation factor 15: consistent marks but not yet ready for promotion. Circ Cardiovasc Genet. 2009;2:209–11. doi: 10.1161/CIRCGENETICS.109.874511. [DOI] [PubMed] [Google Scholar]
- 15.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Jain T, Peshock R, McGuire DK, Willett D, Yu Z, Vega GL, et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–7. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 17.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–9. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 19.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–9. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 20.Abdullah SM, Khera A, Das SR, Stanek HG, Canham RM, Chung AK, et al. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of N-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the Dallas Heart Study) Am J Cardiol. 2005;96:1284–9. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 21.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, et al. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53:284–91. doi: 10.1373/clinchem.2006.076828. [DOI] [PubMed] [Google Scholar]
- 23.Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2009;53:345–52. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med. 2011;30:22–38. doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 25.Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25:3474–86. doi: 10.1002/sim.2299. [DOI] [PubMed] [Google Scholar]
- 26.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 27.D'Agostino R, Nam B. Evaluation of the performance of survival analysis models: discrimination and calibration measures. New York: Elsevier; 2004. [Google Scholar]
- 28.Pencina MJ, D'Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;123:2101–10. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempf T, Sinning JM, Quint A, Bickel C, Sinning C, Wild PS, et al. Growth-differentiation factor-15 for risk stratification in patients with stable and unstable coronary heart disease: results from the Atherogene Study. Circ Cardiovasc Genet. 2009;2:286–92. doi: 10.1161/CIRCGENETICS.108.824870. [DOI] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–33. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 33.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–8. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 34.Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet. 2002;359:2159–63. doi: 10.1016/S0140-6736(02)09093-1. [DOI] [PubMed] [Google Scholar]
- 35.Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, et al. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation. 2007;116:1540–8. doi: 10.1161/CIRCULATIONAHA.107.697714. [DOI] [PubMed] [Google Scholar]
- 36.Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Eur Heart J. 2009;30:2346–53. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]
- 37.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–47. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.