Figure 2.
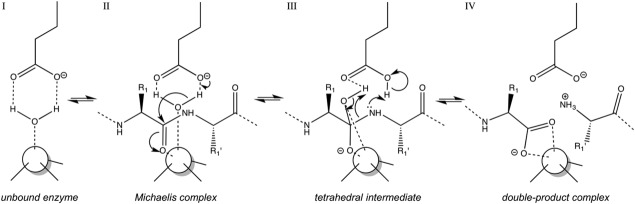
Generally accepted catalytic mechanism of monometallic MPs. The catalytic solvent molecule is bound first to the catalytic metal ion (white sphere) and the general base/acid in the active site in the absence of a peptidic substrate (I). Once the substrate is accommodated in the cleft and the Michaelis complex is formed (II), the polarized solvent molecule attacks the scissile carbonyl group, which leads to the tetrahedral reaction intermediate (III). The latter resolves in scissile bond breakage and double proton transfer to the newly formed α-amino group to render a double-product complex (IV).
