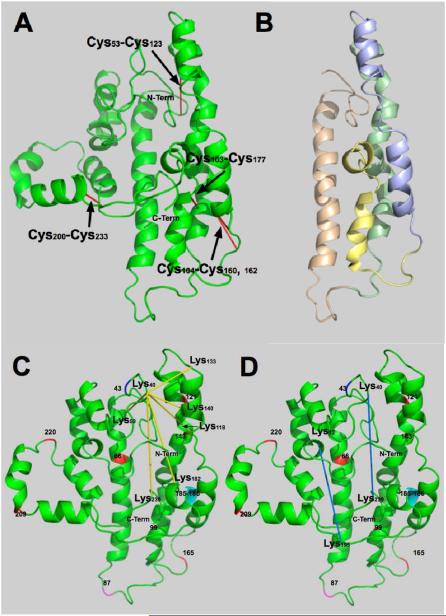
Figure 2. Disulfide bonds and four-helix bundle.
A. Proposed structure for lipid-free apoA-I showing the positions of disulfide bonds that formed spontaneously. B. Conformation shown in A after removing C- and N-termini for clarity: N-terminal, amino acids (AA) 1-43; tan region, AA 44-92, helices 1 and 2; green region, AA 93-130, helices 3-5; blue region, AA 131-166, helices 5 and 6; yellow region, AA 167-187, helix 7; and C-terminal end, AA 188-243, helices 8-10. C. Proposed structure showing the BS3 cross-links radiating from Lys40. D. BS3 cross-links that show the C- and N- terminal ends are close. Cross-links are shown in yellow. Prolines are shown in red, AA 66, 99, 121, 143, 165, 209 and 220. The end of the N-terminal region is shown at AA 43 in blue. Helical region 2 end, AA 87, is shown in magenta. Glycines between helical regions 8 and 9 are shown in cyan.