Abstract
The 20 amino acid N-terminus of the vesicular monoamine transporter 2 (VMAT2) was examined as a regulator of VMAT2 function. Removal of the first 16 or 19 amino acids of the N-terminus resulted in a molecule with reduced ability to sequester [3H]-5HT. A GST-construct of the N-terminus underwent phosphorylation in the presence of PKC at serines 15 and 18. These putative phosphorylation sites were examined for effects on function. Phospho-mimetic substitution of serines 15 and 18 with aspartate in the full-length VMAT2 resulted in reduced [3H]-5HT sequestration and reduced methamphetamine-stimulated efflux of preloaded [3H]-5HT. In contrast, mutation of serines 15 and 18 to alanines maintained intact net substrate sequestration but eliminated methamphetamine-stimulated efflux of pre-accumulated [3H]-5HT. In summary, these data suggest a model in which the VMAT2 N-terminus regulates monoamine sequestration.
Introduction
Among the monoamine neurotransmitter transporters, recent investigations have demonstrated a contribution of the N-terminus to the function of the monoamine plasma membrane transporters (M-PMTs). In response to amphetamine (AMPH) or methamphetamine (METH) treatment, monoamine PMTs enter an efflux-permissive state, releasing cellular monoamine into the extracellular space. This process was demonstrated to involve phosphorylation of the PMT N-termini; for example, in response to AMPH, protein kinase C (PKC) βII, a Ca++-activated PKC-isotype, caused phosphorylation of the dopamine transporter (DAT) N-terminus in human embryonic kidney-293 cells (Khoshbouei et al., 2004, Cervinski et al., 2005, Johnson et al., 2005, Seidel et al., 2005, Fog et al., 2006, Sucic et al., 2010).
The vesicular monoamine transporter 2 (VMAT2) is responsible for sequestering monoamines from the cytosol of monoaminergic cells into vesicular compartments for subsequent exocytotic release (Erickson et al., 1992, Liu et al., 1992a, Liu et al., 1992b). Mice completely lacking VMAT2 die a few days after birth (Fon et al., 1997) whereas hypomorphic mice, expressing severely lowered VMAT2, demonstrate Parkinson's disease (PD) symptoms and pathology later in life (Mooslehner et al., 2001, Caudle et al., 2007). A mutation was identified in the VMAT2 amino acid coding region that severely reduced monoamine transport and correlated with PD symptoms in a Saudi Arabian family (Rilstone et al., 2013). In contrast, VMAT2 gain-of-function promoter haplotypes were shown to correlate with a lower incidence of PD in women (Glatt et al., 2006b). However, despite these findings, evidence correlating polymorphisms in the coding region of the VMAT2 to disease is extremely rare (Glatt et al., 2001, Burman et al., 2004, Glatt et al., 2006a).
The VMAT2 is also a target of METH/AMPH drug action and is being investigated as an intervention target for addiction (Zheng et al., 2006, Crooks et al., 2011). Though the molecular details of the process are not well understood, it is the initial throughway for METH/AMPH-triggered efflux of vesicularly-stored monoamines (Pifl et al., 1995, Sulzer et al., 1995, Sulzer et al., 1996, Takahashi et al., 1997, Sulzer et al., 2005, Partilla et al., 2006). Additionally, striatal-synaptic VMAT2 expression levels are reduced in rats following METH exposure potentially contributing to METH-induced toxicity by compromising cytosolic DA clearance (Eyerman and Yamamoto, 2007, Fleckenstein et al., 2009).
Unlike the longer PMT N-termini, the hVMAT2 is only 20 amino acids (AAs) in length (Fig. 1). It shares 80% homology with the VMAT1 and similar to the monoamine PMTs the N-terminus is putatively localized to the cytosol (Erickson et al., 1992, Liu et al., 1992a, Erickson and Eiden, 1993, Howell et al., 1994, Takahashi and Uhl, 1997, Duerr et al., 1999). Previous investigations have ascribed regulatory functions to the VMAT2 C-terminus (Krantz et al., 1997, Tan et al., 1998, Waites et al., 2001, Li et al., 2005) and the large luminal-loop domain between TMs 1 and 2 (Ahnert-Hilger et al., 1998, Holtje et al., 2000, Ahnert-Hilger et al., 2003, Brunk et al., 2006, Yao and Hersh, 2007). It had been found that photolabels of the two VMAT2 inhibitors tetrabenazine (TBZ) and ketansarin (KSR) derivatized the N-terminus (Sievert and Ruoho, 1997) indicating a possible regulatory role for the N-terminus. The present study further examined the role of the N-terminus in VMAT2 function and found that the N-terminus regulated the level of substrate-sequestration achieved by the VMAT2 as well as the as VMAT2 efflux-response to METH.
Figure 1. Sequence and structural information for hVMAT2.
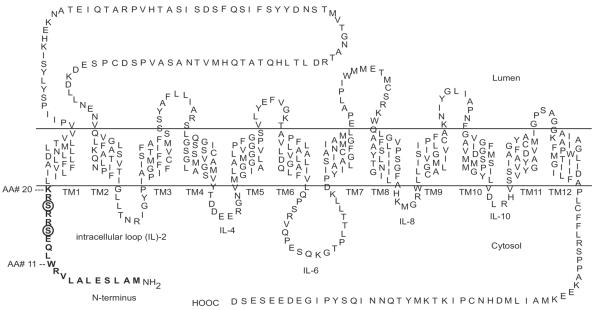
The 20 AA N-terminus is indicated in bold lettering. Putative PKC phosphorylation sites at serines 15 and 18 (referred to in the text) are circled.
Experimental Procedures
Supplies
Cosmic Calf Serum (Hyclone); pGEX vector (Clontech); diolyl phosphatidyl serine (PS; Avanti lipids); Protein kinase C (PKC; a gift from Paul Bertics PhD, Univ. Wisconsin, Madison); protease inhibitors leupeptin, 4-(2-aminoethyl)a benzenesulfonyl fluoride and phenylmethylsulfonyl fluoride (International Chemical and Nuclear); [32P]-γ-ATP (Perkin Elmer); 10,000 kilo dalton (KD) molecular weight cut-off centrifuge filter (Sartorius); polyvinylidene fluoride (PVDF; Millipore); Owl VEP-2 transfer apparatus, 0.1%-Tween casein blocking buffer (ThermoFisher); anti-rabbit secondary antibody (Sigma-Aldrich); horse radish peroxidase (Millipore); the i) peptides ii) the peptide immunogen that was conjugated to keyhole limpet hemocyanin (KLH) in order to generate the pN-term Ab and iii) bovine serum albumin (BSA) derivatized with both phosphorylated and nonphosphorylated forms of the N-terminus were synthesized by Gary Case at the peptide facility, UW-Madison Biotech Center; the phospho-specific N-term antibody (pN-term Ab) was generated by immunization of rabbits at Cocalico; Sulfolink affinity-column (Pierce); [3H]-serotonin (5HT; Perkin Elmer); glass fiber/B filters (Whatman).
Cell Culture, Transfections, Plasmid Constructs
COS-cells were cultured in Dulbecco's modified eagles medium, 10% Cosmic Calf Serum at 5% CO2 at 37°C. One day prior to transfection, cells were divided to obtain 90–100% confluency on the day of transfection. Cells were trypsinized, washed one time in phosphate buffered saline (PBS) and resuspended in 500μl of `cytomix' (van den Hoff et al., 1992) buffer containing a given amount of DNA. Cell/DNA mixture was transferred to a 4mm cuvette and electroporated at 950μF and 226 mV. Cuvettes were placed in a 37°C 5% CO2 incubator 15 min prior to transferring cells to media to allow the cells to recover from electroporation. Cells were usually used (“harvested”) within 24 hours of transfection. The WT human VMAT2 construct has been previously described (Thiriot and Ruoho, 2001). Basically the hVMAT2 construct was inserted into pcDNA3.1 (−) at Xho1 and HIII sites. A Flag and a 6-histidine epitope were placed sequentially on the C-terminus. Finally, glycosylation sites were removed as described previously (Thiriot and Ruoho, 2001), and an hemagglutinin (HA) epitope was inserted in the first luminal loop between TMs 1 and 2. The S15/18A and S15/18D N-terminus VMAT2 mutants were created from this construct through QuickChange mutagenesis. Mutagenized constructs were sequenced (at the UW Biotech Center) to confirm creation of the desired sequences.
In Vitro Kinase Assays and Western Immunoblots
VMAT2 N-terminal WT, S15A, S18A and S15/18A GST constructs were obtained using the inducible pGEX vector system. The constructs were transformed into BL21 bacteria from which the final glutathione-S-transferase (GST) fusion-protein was extracted with glutathione and PBS washed following BL21 growth and lysis according to the manufacturers protocol. Confirmation of the correct size of the fusion protein as well as determination of concentration was performed by SDS-PAGE (sodium dodecyl sulfate - polyacrylamide gel) separation followed by Coomassie staining and analysis with ImageJ software to quantify band size and determine final protein concentrations of the various GST-constructs used.
For the SH-SY5Y lysate experiment that examined N-terminus phospho-detection by the pN-term Ab (Fig. 3A), SH-SY5Y concentrated lysate was obtained by lysis of SH-SY5Y cells in sucrose HEPES (SH) buffer (0.03M sucrose, 10mM HEPES pH 7.2) and protease inhibitors leupeptin, 4-(2-aminoethyl) benzenesulfonyl fluoride and phenylmethylsulfonyl fluoride). Cells were suspended in 500μl of buffer and vigorously passaged through a 30.5 guage needle/syringe five times. Following removal of nuclear debris by five minute room temperature low-speed centrifugation the homogenate was centrifuged 30–60 min. in a 10,000KD molecular weight cut-off centrifuge filter 4°C at 4,000 rpm resulting in a 10 fold volume concentration of the original lysate. 2μl of this was used immediately per sample. The kinase buffer for PKC and SH-SY5Y lysate assays consisted of 4mM HEPES pH 7, 2.5mM magnesium chloride (MgCl2), 100μM calcium chloride (CaCl2), 2.5mM ATP. Kinase assays were initiated by addition of 0.1 U PKC or concentrated SH-SY5Y lysate to 20μl of sample containing 250 pmoles of GST construct. The sample was moved from an ice bath to 37°C water bath for 10 minutes (in the presence of various drugs described in Fig. 3A, B) then halted on ice. Samples were subsequently processed for SDS-PAGE immunoblot analysis.
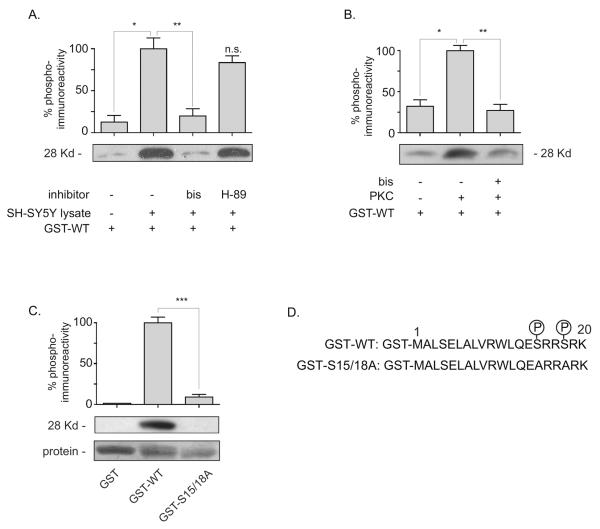
Figure 3. Phosphorylation of a GST-N-terminus construct of VMAT2 by SH-SY5Y cell lysate or PKC.
A. SH-SY5Y cell concentrate was assessed for phosphorylation activity directed at 250 pmoles of the GST-WT N-terminus construct in the presence of ATP and MgCl2. Inhibitors of PKC and PKA were tested for inhibition of lysate-kinase activity at concentrations 300X greater then their IC50s [PKC, bis (3μM); PKA, H-89 (3μM)]. *,**: P=.0012, .0021. Samples were separated on 12% SDS-Tris-acrylamide gels and immunobloted with the pN-term Ab described in Experimental Procedures. Graph is mean ± S.E. of four experiments with lysate + GST-WT set at 100%. n.s. not statistically different from GST-WT + lysate condition. B. PKC phosphorylation of the N-terminus. ± PKC (0.1 U) was added to 20μl samples containing 250 pmoles of GST-WT N-terminus construct. Samples analyzed same as A. Graph is mean ± S.E. of three experiments with PKC + GST-WT set at 100%. *,**: P=.0027, .0018. C. PKC was added to 250 pmoles of GST or WT or A2 (serines 15 and 18 mutated to alanines) VMAT2 GST-constructs of the N-terminus (Experimental Procedures). Samples were analyzed same as in A and B. Graph is mean ± S.E. of three experiments normalized to respective protein inputs with PKC + GST-WT set at 100%. ***: P=.0003. D. Sequence of the GST-N-terminus constructs with putative phosphorylation sites at serines 15 and 18 indicated. Molecular weight based on GST (26Kd) and 20 AA N-terminus (2Kd).
For the Western immunoblots, 20μl of assay sample (PKC or SH-SY5Y lysate samples) were adjusted up to 25μl with 5μl 5X sample buffer and separated on 12% acrylamide low-cross-linked separating gels (0.06% bis-acrylamide) using mid-range prestained molecular weight markers (Biorad) to follow separation. Protein was transferred to methanol presoaked PVDF in an Owl VEP-2 transfer apparatus. PVDF membranes were then blocked in casein blocking buffer (ThermoFisher) supplemented with 0.1%-tween 1 for hour and then exposed to pN-term Ab (Experimental Procedures) overnight at 4°C on a rotating platform at an antibody dilution of 1:3,000. Membranes were washed 3× 10 minutes in 0.1%-tween PBS and exposed to horse radish peroxidase - conjugated anti-rabbit secondary antibody (diluted at 1:150000) in the same casein buffer for 1 hour at room temperature. PVDF membranes were again washed in PBS-tween buffer 3× 10 minutes and exposed to horse radish peroxidase substrate for 5 minutes according to manufacturers directions, exposed to X-ray film and developed.
Antisera Affinity-Column Purification of Phospho-Specific N-terminus VMAT2 Ab (pN-term Ab)
A total of three different carrier molecules, fused or conjugated to AA sequences of the VMAT2 N-terminus, were used to create and assess the immunoreactivity of an antibody generated to recognize the VMAT2 N-terminus phosphorylated at serines 15 and 18 (pN-term Ab). To create an immunogen against the phosphorylated N-terminus, keyhole limpet hemocyanin (KLH) was derivatized with an N-terminal cysteine construct (at multiple positions) using a nine AA VMAT2 N-terminus peptide that was completely phosphorylated on both serines (hVMAT2 serines 15 and 18): CRWLQW[pS]RR[pS]RK. BSA was similarly conjugated (at multiple positions) to the N-terminal methionine residue of the 20 AA N-terminus. A carrier BSA molecule was derivatized for both phosphorylated and nonphosphorylated forms of the N-terminus. A third carrier molecule was the VMAT2 N-terminal GST fusion-protein previously described. The KLH-conjugated peptide was suspended in PBS and injected into rabbits. The second boost anti-sera was fractionated with saturated ammonium sulfate to precipitate out antibody that was centrifuged at 1500 × g for 10 min to isolate the protein pellet that was then resuspended in 0.5ml of 0.9% sodium chloride and dialyzed in 10mM PBS pH 7.5 for 48 hours with frequent PBS changes to remove the ammonium sulfate. Dialyzed material was then purified on a Sulfolink affinity-column against the BSA-conjugated phospho-peptide from the VMAT2 N-terminus in which serines 15 and 18 had been completely derivatized with phosphates. The isolated material was washed in the manufacturer's `Sample' buffer and eluted with 8ml glycine buffer (100mM, pH 2.5–3). The resulting antibody was assessed for immunoreactivity against the BSA-coupled phosphorylated VMAT2 N-terminus in an enzyme-linked immunosorbent based assay in which increasing amounts of nonphosphorylated or phosphorylated peptide were used to assess the binding-affinity of the antibody. The antibody was also assessed for immunoreactivity against the phosphorylated N-terminus by western immunoblotting: the phosphorylated N-terminus coupled to BSA was immuno-detectable at 1 pmole whereas 100 pmoles of nonphosphorylated GST-N-terminus fusion protein could not be detected.
Bioinformatics; Kinase Site Identification; Statistics
The N-terminus VMAT2 sequence was submitted to the website: http://www.cbs.dtu.dk/services/NetPhos/. Regions giving a high-score result (score > .80) were interpreted as indication of a potential phosphorylation site. Two-tailed P values were obtained using the t tests (and nonparametric tests) function of GraphPad Prism.
[3H] 5HT Sequestration and Efflux Assays and [3H] TBZOH Binding Assays
COS-cell homogenates, containing cellular compartments (endosomes and lysosomes; also referred to as “COS-membranes”) capable of ATP/MgCl2 reserpine (RSP) inhibitable substrate (e.g. [3H]-5HT) sequestration (i.e. “transport”) were obtained as follows: hVMAT2 transfected COS-cells were trypsinized from plates, washed 3X in PBS to remove all the trypsin and resuspended in 4°C sucrose HEPES (SH: 0.3M sucrose, 10mM HEPES pH 7.2) buffer. Cells were passed 5X at high pressure through a 30.5 guage needle. Nonlysed cells were removed with 300 × g 5min centrifugation.
For the sequestration assays COS-membrane preparations were supplemented with 10mM MgCl2, 10mM ATP and 30nM [3H]-5HT and incubated at 32°C for the periods of time indicated in the figure legend. Membranes were then immediately filtered onto polyethylenimine presoaked glass fiber/B filters on a Brandel filtration apparatus and washed with cold SH buffer - free of 5HT, ATP and MgCl2 and counts per minute (CPM) or decays per minute (DPM) were obtained with a liquid scintillation counter and converted to pmoles of [3H]-5HT retained and normalized to μg of protein per sample per minute of sequestration time (Fig. 2A) or [3H]-tetrabenazine-OH (TBZ-OH) bound per sample per minute (Fig. 2B, see binding assays paragraph). Reserpine inhibited (i.e., VMAT2 specific) [3H]-5HT sequestration was not observed in non-VMAT2 transfected COS-membranes (data not shown).
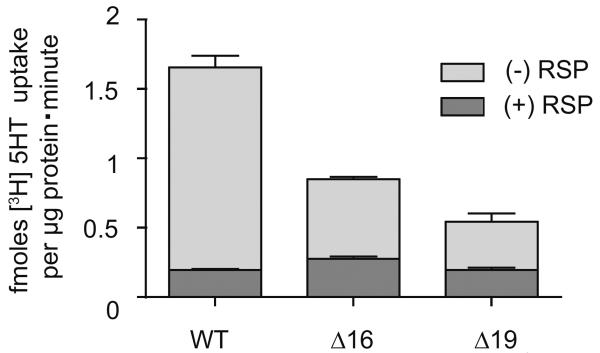
Figure 2. Substrate accumulation is lowered in N-terminus truncated VMAT2 COS-membranes.
Removal of N-terminus AAs 1–16 (Δ16) or 19 (Δ19) reduces net substrate sequestration by the VMAT2. 10μl crude-membranes were obtained from COS-cells transfected with WT or Δ16 or Δ19 hVMAT2 constructs and assessed for accumulation of [3H]-5HT for 10 minutes in the presence of ATP and MgCl2 and normalized to μg protein per sample. Lower dark bar represents nonspecific [3H]-5HT accumulation in the presence of reserpine (RSP). Data are mean ± S.E. of values obtained from two separate experiments done in triplicate (n=6).
Efflux assays (Fig. 2C), were essentially a modification of the transport assays with an additional step: prior to being diluted up in buffer for the efflux step, 10μl COS-membranes were first subjected to an uptake (i.e. `transport') step for 20 minutes in order to sequester [3H]-5HT. This was carried out in SH buffer with 2.5mM ATP and 2.5mM MgCl2 and 5mM KCl. Following this step, these membranes were diluted 40X with 400μl of efflux buffer (transport buffer with 200nM CaCl2 lacking [3H]-5HT, ATP and MgCl2). The efflux step was allowed to proceed for a time course (up to nine minutes) at 30°C after which membranes were immediately filtered and washed as in the sequestration assays. Efflux was expressed as the percent of [3H]-5HT that was retained in the filter trapped COS-membranes using the following method of analysis. First, each value was corrected to account for nonspecific [3H]-5HT accumulated during the preloading phase - in the presence of the VMAT inhibitor reserpine (RSP). This RSP value was subtracted from each value. Values measured at the zero time point (termed total) typically fell within the range of 5,000–15,000 decays per minute (DPM) of accumulated [3H]-5HT per sample and a RSP insensitive [3H]-5HT background value of 300–1000 DPM. Second, the [3H]-5HT retention value at each time point in the presence or absence of METH was normalized to the [3H]-5HT transport value obtained at 0 minutes and plotted.
For binding assays (Fig. 4), 50μl of COS-membranes were incubated 45 minutes at 30°C in the presence of 1, 5, 10, 20, 40 and 80nM [3H]-tetrabenazine-OH (TBZ-OH). Specific [3H]-TBZ-OH binding was assessed at each [3H]-TBZ-OH concentration in the presence of 20μM nonradioactive TBZ. Incubations proceeded for 45 minutes, vesicles were filtered on a Brandel filtration apparatus and then washed with cold SH buffer as in the sequestration and efflux assays. Two values were obtained for each specific and nonspecific point at each concentration of [3H]-TBZOH used (1, 5, 10, 20, 40 and 80nM). The crude-membranes were filtered on a Brandel filtration apparatus. The amount of radioactivity bound to filters was determined by [3H] CPM or DPM measurement with a liquid scintillation counter. For each concentration of [3H]-TBZ-OH a specific bound value was obtained by subtracting [3H]-TBZOH in the presence of 20μM nonradioactive TBZ from [3H]-TBZOH alone. This value was converted to pmoles [3H]-TBZ-OH bound per sample. Bmax and Kd values for [3H]-TBZ-OH binding were determined with GraphPad Prism software.
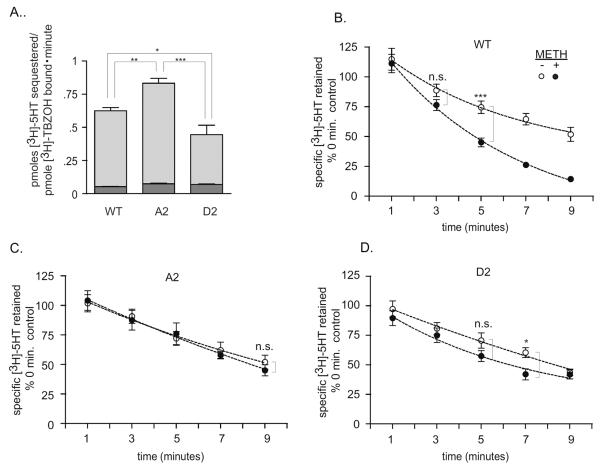
Figure 4. Substrate accumulation is lowered in D2 COS-membranes. METH-stimulated [3H]-5HT efflux is reduced in A2 containing COS-membranes.
A. Comparison of [3H]-5HT sequestration in WT, A2 and D2 crude COS-membranes. 50μl crude-membranes were obtained from COS-cells transfected with WT hVMAT2, or hVMAT2 with N-terminus serines 15 and 18 mutated to alanines (A2) or aspartates (D2), pre-incubated at 30°C for 10 minutes with ATP and MgCl2, supplemented with 30 nM [3H]-5HT and assessed for accumulation [3H]-5HT for 3 minutes in the presence of ATP and MgCl2 and normalized to the Bmax of specific [3H]-TBZOH binding (a relative measure of the amount of VMAT2 present, Table 1). Lower dark bar represents nonspecific [3H]-5HT accumulation in the presence of RSP. Lighter upper bar shows specific [3H]-5HT accumulation. Data shown is mean ± S.E. (excluding values > 3 standard deviations from mean) of values obtained from three experiments done in duplicate (WT n=5, A2 n=6, D2 n=6). **: P = 0.0085; *: P = 0.0413; ***: P = 0.0008. B–D. Comparison of specific [3H]-5HT retained in WT, A2 and D2 COS-membranes at indicated minutes following addition of efflux buffer −/+30μM METH. Data were obtained using 10 μl of hVMAT2-expressing COS-membranes. Data is mean ± S.E. of values from two experiments for WT and D2 and three experiments for A2 done in triplicate (excluding values > 3 standard deviations from mean). ***: P = 0.001; *: P=0.0151. not statistically significant (n.s.). Lines generated with GraphPad Prism one phase exponential decay function.
Results
(Sievert and Ruoho, 1997)A prior investigation indicated that removal of the first 18 AAs of the rat VMAT2 N-terminus reduced net substrate sequestration (Tan et al., 1998). Thus, to further determine the effect of the N-terminus on VMAT2 function, hVMAT2 mutant constructs lacking the first 16 or 19 AAs (Δ16, Δ19) were transfected into COS-cells and tested for net substrate accumulation. De-nucleated crude membranes were obtained from COS-cells transfected with hVMAT2 plasmid (Experimental Procedures) and assessed for accumulation of substrate in the presence of ATP, MgCl2 and radiolabelled monoamine substrate ([3H]-5HT). As with the Δ18 mutant (Tan et al., 1998), crude membrane accumulation of [3H]-5HT was significantly altered by removal of the N-terminus (Fig. 2).
A screen of the N-terminus predicted potential phosphorylation by PKA and PKC at serines 15 and 18 (Experimental Procedures). To examine if these kinases were capable of phosphorylating the N-terminus it was hypothesized that kinase activity targeting the VMAT2 N-terminus would be contained in the lysate concentrated from a cell-line expressing endogenous VMAT2. SH-SY5Y cells are a human neuroblastoma cell line that express endogenous VMAT2 (Vaughan et al., 1995, Pan et al., 2005). Therefore GST-VMAT2 N-terminus fusion-protein was exposed to concentrated SH-SY5Y cell-lysate in the presence of ATP and Mg++ for 30 minutes at 30°C. Phospho-incorporation at serines 15 and/or 18 on the GST-fusion protein was then determined using an antibody that had been specifically generated against the phosphorylated VMAT2 N-terminus (pN-term, Experimental Procedures). Shown in Fig. 3A, the pN-term antibody detected phosphorylated N-terminus in the presence of ATP, Mg++ and concentrated SH-SY5Y lysate (lane 2). Inhibitors of kinases PKC (bis) and PKA (H-89) were tested at concentrations 300 fold higher then their reported IC50s. Only the PKC inhibitor bis-indolylmaleimide (bis) significantly inhibited phospho-detection by the pN-term Ab, indicating that the VMAT2 N-terminus was potentially a target of PKC (Fig. 3A). To verify PKC phosphorylation of the N-terminus, purified PKC was added to GST-VMAT2 N-terminus fusion-protein in the presence of ATP and Mg++ for 30 minutes at 30°C. As with the SH-SY5Y lysate, purified PKC also lead to bis-inhibited phospho-incorporation on the N-terminus detected by the pN-term Ab (Fig. 3B). To further verify serine 15 and/or 18 directed PKC activity, three different GST-constructs were assessed for phosphorylation. Incubation of the GST-N-terminus construct with PKC lead to phosphorylation of the WT-construct (detected by the pN-term Ab) but not GST alone or a construct that had serine 15 and 18 mutated to alanine (S15/18A) indicating specific phosphorylation at these residues (Fig. 3C).
Because reduced sequestration of substrate was observed with removal of the N-terminus, it was possible that mutating serines 15 and 18 would also change VMAT2 function. Thus the effect of aspartate (D2) or alanine (A2) substitutions at these sites was examined. However, it was possible that the reduction in sequestration observed with the Δ16 and Δ19 truncation mutants reflected reduced biosynthesis of these mutants. Thus, a measure of VMAT2 expression was needed. As a measure of transporter expression, each transporter-type was normalized to the Bmax obtained for specific binding of the VMAT2 ligand [3H]-TBZOH. The affinity of [3H]-TBZOH binding (Kd) was not substantially altered between the three VMAT2-transfects (Table 1) providing evidence to support the proper folding of A2 and D2. When the amount of [3H]-5HT sequestered in 3 minutes was normalized to the [3H]-TBZOH Bmax value for each transporters expression, A2-membranes demonstrated an increased ability to accumulate [3H]-5HT relative to WT-membranes, whereas D2-membranes lost accumulation relative to both WT and A2 membranes (Fig. 4A).
Table 1.
Average [3H]-tetrabenazine-OH ([3H]-TBZOH) VMAT2 binding affinities and Bmax
| Kd (nM) ± SE | Bmax ± SE** | |
|---|---|---|
| WT VMAT2 | 28.3 ± 3.0 | 100 ± 8.2 |
| A2 VMAT2 | 22.5 ± 3.3 n.s. | 62.2 ± 4.2* |
| D2 VMAT2 | 22.6 ± 1.0 n.s. | 57.9 ± 3.6*✠ |
Values are averages from three experiments.
Bmax values are normalized to WT VMAT2 set to 100%. n.s. no statistical difference to WT.
P=.0149 and .009 for WT vs A2, and WT vs D2 respectively.
no statistical difference with A2.
While it appeared that the mutations of the N-terminus had altered VMAT2 transport activity, the VMATs also mediate efflux of vesicularly sequestered monoamines in response to efflux agents such as AMPH and METH (Rudnick and Wall, 1992, Sulzer et al., 1995, Mosharov et al., 2003, Sulzer et al., 2005, Partilla et al., 2006). Thus, a possible explanation for the changes in net substrate sequestration by the mutants was that they had triggered changes in the release of sequestered [3H]-5HT from the VMAT2-transfected COS-membranes. To test this, hVMAT2 expressing membranes were subjected to a release assay: Following a pre-incubation period for [3H]-5HT accumulation, [3H]-5HT, ATP and MgCl2 concentrations were diluted forty-fold to minimize inward transport of substrate. The amount retained in membranes was measured at 1, 3, 5, 7 and 9 minutes following addition of the dilution buffer. Addition of an efflux-agent to WT VMAT2 membranes (30μM METH) resulted in la greater loss of sequestered [3H]-5HT relative to untreated WT membranes (Fig. 4B). The greater level of sequestration achieved by the A2 mutant and the reduced level achieved by the D2 mutant suggested that they would undergo enhanced and diminished loss of pre-accumulated [3H]-5HT, respectively in the presence of METH. Surprisingly, METH-stimulated [3H]-5HT efflux was not observed, at any time, in A2 membranes. Some loss of pre-accumulated [3H]-5HT in the presence of METH was observed in D2 membranes. However this was greatly reduced compared to loss from WT membranes, perhaps due to a decreased ability of the D2 mutant to accumulate substrate prior to the efflux experiment.
Discussion
In the present study, the VMAT2 N-terminus was characterized for its influence on transporter function. Removal of 16 or 19 AAs of the N-terminus reduced net substrate sequestration (Fig. 2) consistent with a previous report of N-terminus removal (Tan et al., 1998). A GST-construct of the N-terminus underwent PKC-dependent phosphorylation at serines 15 and/or 18 (Fig. 3). PKA was also a predicted kinase for serine 15, however, no significant inhibition of phosphorylation was observed in the SH-SY5Y lysate using the PKA inhibitor H-89 at a concentration 300-fold in excess of it's IC50. It is possible that the phosphorylation site may not have been accessible to PKA on the structure of the GST-linked N-terminus. Therefore, targeting of the N-terminus by PKA cannot be completely ruled out. The conclusion from this part of the study is that serines 15 and/or 18 can be targeted on the GST-N-terminus construct by PKC in vitro.
It was then determined what the effect of phospho- and non phospho-mimetic substitutions would be on VMAT2 function. Since it was possible that expression of the Δ16 or Δ19mutants had been altered by truncation of the N-terminus, [3H]-TBZOH binding was used as a measure of total VMAT2 present for the WT, A2 and D2 sequestration samples. No significant Kd variation was detected for [3H]-TBZOH binding between these VMAT2 forms, demonstrating that the affinity for ligand was not significantly altered between the VMAT2 forms (Table 1). This was of particular importance for measuring the difference between A2 and D2 sequestration since these mutants achieved the highest and lowest [3H]-5HT sequestration levels respectively yet demonstrated no significant difference between their [3H]-TBZOH Kds (Fig. 4A, Table 1). No significant difference was observed between the Bmax values of specific [3H]-TBZOH binding for A2 and D2 mutants (as a measure of VMAT2 expression - Table 1). Therefore it is reasonable to conclude that the difference in achieved sequestration between the A2 and D2 mutants reflects a difference in VMAT2 function. The use of [3H]-TBZOH Bmax to measure transporter expression did identify a consistent decrease in VMAT2 expression for both the A2 and D2 mutants relative to WT VMAT2 (Table 1 ). Thus, use of [3H]-TBZOH enabled normalization for the reduced expression of these mutants.
As previously reported (Pifl et al., 1995, Sulzer et al., 1995, Mosharov et al., 2003, Sulzer et al., 2005, Partilla et al., 2006) treatment with an efflux agent, in this case METH, triggered VMAT2-mediated efflux of sequestered substrate (Fig. 4B). Surprisingly, mutation of serines 15 and 18 to alanines or aspartates respectively eliminated or reduced substrate efflux in response to treatment with METH, over the nine minute time-course, indicating that the N-terminus mediates the VMAT2 efflux function. As described in the Introduction, N-terminus directed PKC activity mediates efflux by the monoamine PMTs. Additionally, disrupting the interaction of SERT with negatively charged phosphatidylinositol-4,5-bisphosphate (PIP2) head groups triggered SERT-PIP2 dissociation, reducing amphetamine-triggered SERT-mediated currents (Buchmayer et al., 2013), suggesting that it would be informative to determine whether positively charged regions at the VMAT2 cytosol interface, including within the N-terminus, interact with PIP2. Further study is needed to uncover the molecular details of how altering the molecular-chemistry of the N-terminus alters VMAT2 function. Nevertheless these data support a role for N-terminus as a regulator of VMAT2 function: N-terminus modification directly influenced the level of sequestration achieved by the VMAT2 as well as the VMAT2 efflux-response to METH and potentially other efflux agents.
From these data it can be speculated that METH-treatment alters the net positive charge on the N-terminus via phosphorylation, triggering the release of monoamine. However, while the GST-construct of the N-terminus underwent PKC-dependent phosphorylation at serines 15 and/or 18, a clear demonstration with the pN-term Ab that the full-length transporter was phosphorylated in situ was problematic (possibly due to masking of N-terminus phosphorylation sites by an undetermined intra- or intermolecular interaction). Therefore, additional approaches to detecting N-terminus phosphorylation may be required. A recent example from the literature, being the use of mass-spectroscopy to detect phosphorylation of the DAT N-terminus (Foster et al., 2012).
Conclusions
These data support the conclusion that the VMAT2 N-terminus regulates the sequestration of monoamine as well as its METH-triggered efflux from storage sites in VMAT2-transfected crude-membranes.
Acknowledgments
The authors wish to thank Drs. Michael Sievert and David Thiriot for generating the full-length and GST-fusion protein VMAT2 constructs and Dr. Arindam Pal for generation of the pN-term Ab.
This work was supported by an American Heart Association Predoctoral Fellowship (BT), the University of Wisconsin Cardiovascular Research Training Grant (BT) and the UW McPherson Eye Research Institute Edwin and Dorothy Gamewell Retina Research Foundation Professorship (AER)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors contribution Research design: Torres, Ruoho
Conducted experiments: Torres
Contributed analytic tools: Torres, Ruoho
Performed data analysis: Torres, Ruoho
Contributed to writing the manuscript: Torres, Ruoho
References
- Ahnert-Hilger G, Holtje M, Pahner I, Winter S, Brunk I. Regulation of vesicular neurotransmitter transporters. Rev Physiol Biochem Pharmacol. 2003;150:140–160. doi: 10.1007/s10254-003-0020-2. [DOI] [PubMed] [Google Scholar]
- Ahnert-Hilger G, Nurnberg B, Exner T, Schafer T, Jahn R. The heterotrimeric G protein Go2 regulates catecholamine uptake by secretory vesicles. Embo J. 1998;17:406–413. doi: 10.1093/emboj/17.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk I, Blex C, Rachakonda S, Holtje M, Winter S, Pahner I, Walther DJ, Ahnert-Hilger G. The first luminal domain of vesicular monoamine transporters mediates G-protein-dependent regulation of transmitter uptake. J Biol Chem. 2006;281:33373–33385. doi: 10.1074/jbc.M603204200. [DOI] [PubMed] [Google Scholar]
- Buchmayer F, Schicker K, Steinkellner T, Geier P, Stubiger G, Hamilton PJ, Jurik A, Stockner T, Yang JW, Montgomery T, Holy M, Hofmaier T, Kudlacek O, Matthies HJ, Ecker GF, Bochkov V, Galli A, Boehm S, Sitte HH. Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11642–11647. doi: 10.1073/pnas.1220552110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman J, Tran CH, Glatt C, Freimer NB, Edwards RH. The effect of rare human sequence variants on the function of vesicular monoamine transporter 2. Pharmacogenetics. 2004;14:587–594. doi: 10.1097/00008571-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Zheng G, Vartak AP, Culver JP, Zheng F, Horton DB, Dwoskin LP. Design, synthesis and interaction at the vesicular monoamine transporter-2 of lobeline analogs: potential pharmacotherapies for the treatment of psychostimulant abuse. Curr Top Med Chem. 2011;11:1103–1127. doi: 10.2174/156802611795371332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci. 1999;19:72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, Eiden LE. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993;61:2314–2317. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Eiden LE, Hoffman BJ. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. Journal of neurochemistry. 2007;103:1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology. 2009;56(Suppl 1):133–138. doi: 10.1016/j.neuropharm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Foster JD, Yang JW, Moritz AE, Challasivakanaka S, Smith MA, Holy M, Wilebski K, Sitte HH, Vaughan RA. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. The Journal of biological chemistry. 2012;287:29702–29712. doi: 10.1074/jbc.M112.367706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt C, Almonte M, Taylor T, Edwards RH, Freimer N, Tanner C. Structural variants in the vesicular monoamine transporter do not contribute to sporadic Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2006a;21:426–427. doi: 10.1002/mds.20798. [DOI] [PubMed] [Google Scholar]
- Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, Risch N, Freimer NB. Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes. Nature genetics. 2001;27:435–438. doi: 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- Glatt CE, Wahner AD, White DJ, Ruiz-Linares A, Ritz B. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006b;15:299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansra N, Arya S, Quick MW. Intracellular domains of a rat brain GABA transporter that govern transport. J Neurosci. 2004;24:4082–4087. doi: 10.1523/JNEUROSCI.0664-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtje M, von Jagow B, Pahner I, Lautenschlager M, Hortnagl H, Nurnberg B, Jahn R, Ahnert-Hilger G. The neuronal monoamine transporter VMAT2 is regulated by the trimeric GTPase Go(2) J Neurosci. 2000;20:2131–2141. doi: 10.1523/JNEUROSCI.20-06-02131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M, Shirvan A, Stern-Bach Y, Steiner-Mordoch S, Strasser JE, Dean GE, Schuldiner S. Cloning and functional expression of a tetrabenazine sensitive vesicular monoamine transporter from bovine chromaffin granules. FEBS Lett. 1994;338:16–22. doi: 10.1016/0014-5793(94)80108-8. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz DE, Peter D, Liu Y, Edwards RH. Phosphorylation of a vesicular monoamine transporter by casein kinase II. J Biol Chem. 1997;272:6752–6759. doi: 10.1074/jbc.272.10.6752. [DOI] [PubMed] [Google Scholar]
- Li H, Waites CL, Staal RG, Dobryy Y, Park J, Sulzer DL, Edwards RH. Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron. 2005;48:619–633. doi: 10.1016/j.neuron.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992a;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roghani A, Edwards RH. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proc Natl Acad Sci U S A. 1992b;89:9074–9078. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS, Emson PC. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol Cell Biol. 2001;21:5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Gong LW, Khanna B, Sulzer D, Lindau M. Intracellular patch electrochemistry: regulation of cytosolic catecholamines in chromaffin cells. J Neurosci. 2003;23:5835–5845. doi: 10.1523/JNEUROSCI.23-13-05835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Xie W, Jankovic J, Le W. Biological effects of pramipexole on dopaminergic neuron-associated genes: relevance to neuroprotection. Neurosci Lett. 2005;377:106–109. doi: 10.1016/j.neulet.2004.11.080. [DOI] [PubMed] [Google Scholar]
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319:237–246. doi: 10.1124/jpet.106.103622. [DOI] [PubMed] [Google Scholar]
- Pifl C, Drobny H, Reither H, Hornykiewicz O, Singer EA. Mechanism of the dopamine-releasing actions of amphetamine and cocaine: plasmalemmal dopamine transporter versus vesicular monoamine transporter. Mol Pharmacol. 1995;47:368–373. [PubMed] [Google Scholar]
- Rilstone JJ, Alkhater RA, Minassian BA. Brain dopamine-serotonin vesicular transport disease and its treatment. The New England journal of medicine. 2013;368:543–550. doi: 10.1056/NEJMoa1207281. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Wall SC. p-Chloroamphetamine induces serotonin release through serotonin transporters. Biochemistry. 1992;31:6710–6718. doi: 10.1021/bi00144a010. [DOI] [PubMed] [Google Scholar]
- Seidel S, Singer EA, Just H, Farhan H, Scholze P, Kudlacek O, Holy M, Koppatz K, Krivanek P, Freissmuth M, Sitte HH. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- Sievert MK, Ruoho AE. Peptide mapping of the [125I]Iodoazidoketanserin and [125I]2-N-[(3'-iodo-4'-azidophenyl)propionyl]tetrabenazine binding sites for the synaptic vesicle monoamine transporter. J Biol Chem. 1997;272:26049–26055. doi: 10.1074/jbc.272.41.26049. [DOI] [PubMed] [Google Scholar]
- Sucic S, Dallinger S, Zdrazil B, Weissensteiner R, Jorgensen TN, Holy M, Kudlacek O, Seidel S, Cha JH, Gether U, Newman AH, Ecker GF, Freissmuth M, Sitte HH. The N terminus of monoamine transporters is a lever required for the action of amphetamines. J Biol Chem. 2010;285:10924–10938. doi: 10.1074/jbc.M109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Sulzer D, St Remy C, Rayport S. Reserpine inhibits amphetamine action in ventral midbrain culture. Mol Pharmacol. 1996;49:338–342. [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Uhl G. Murine vesicular monoamine transporter 2: molecular cloning and genomic structure. Brain Res Mol Brain Res. 1997;49:7–14. doi: 10.1016/s0169-328x(97)00116-2. [DOI] [PubMed] [Google Scholar]
- Tan PK, Waites C, Liu Y, Krantz DE, Edwards RH. A leucine-based motif mediates the endocytosis of vesicular monoamine and acetylcholine transporters. J Biol Chem. 1998;273:17351–17360. doi: 10.1074/jbc.273.28.17351. [DOI] [PubMed] [Google Scholar]
- Thiriot DS, Ruoho AE. Mutagenesis and derivatization of human vesicle monoamine transporter 2 (VMAT2) cysteines identifies transporter domains involved in tetrabenazine binding and substrate transport. J Biol Chem. 2001;276:27304–27315. doi: 10.1074/jbc.M103947200. [DOI] [PubMed] [Google Scholar]
- van den Hoff MJ, Moorman AF, Lamers WH. Electroporation in `intracellular' buffer increases cell survival. Nucleic Acids Res. 1992;20:2902. doi: 10.1093/nar/20.11.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan PF, Peers C, Walker JH. The use of the human neuroblastoma SH-SY5Y to study the effect of second messengers on noradrenaline release. Gen Pharmacol. 1995;26:1191–1201. doi: 10.1016/0306-3623(94)00312-b. [DOI] [PubMed] [Google Scholar]
- Waites CL, Mehta A, Tan PK, Thomas G, Edwards RH, Krantz DE. An acidic motif retains vesicular monoamine transporter 2 on large dense core vesicles. J Cell Biol. 2001;152:1159–1168. doi: 10.1083/jcb.152.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hersh LB. The vesicular monoamine transporter 2 contains trafficking signals in both its N-glycosylation and C-terminal domains. J Neurochem. 2007;100:1387–1396. doi: 10.1111/j.1471-4159.2006.04326.x. [DOI] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Crooks PA. Vesicular monoamine transporter 2: role as a novel target for drug development. Aaps J. 2006;8:E682–692. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]