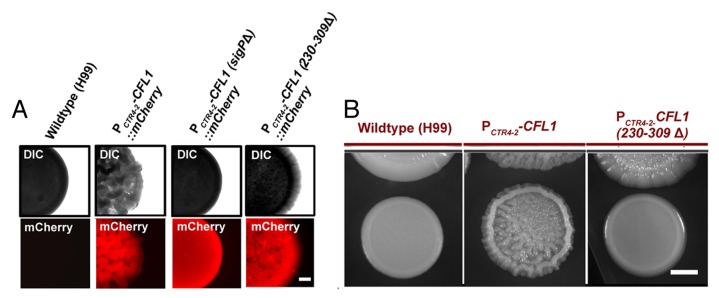
Figure 1. Secretion and the C-terminal SIGC domain are important for Cfl1’s multi-functions. (A) The expression of the full length and the truncated Cfl1-mCherry proteins [CFL1(sigPΔ) and CFL1(230–309Δ)] was driven by the promoter of the copper transporter CTR4 (PCTR4–2). Indicated strains were grown on YPD agar medium supplemented with the inducer BCS (copper chelator) at 200 μM. Cells were cultured at 22°C for 3 d before being examined under a fluorescence stereoscope. The degree in colony wrinkledness reflects the strength in cell-cell adhesion and the complexity in colony morphology (or biofilm). The deletion of the N-terminal signal peptide (sigPΔ) or the SIGC domain (230–309Δ) did not significantly affect the protein expression level based on their fluorescence intensity. The C. neoformans strain expressing CFL1(sigPΔ) did not form biofilm colonies while the strain expressing the SIGC truncated allele CFL1(230–309Δ) showed decreased wrinkledness in colony morphology. Scale bar: 1 mm. (B) The donor strain expressing the SIGC truncated CFL1 allele failed to evoke the nearby wildtype recipient to form a structured biofilm colony. The confrontation assay was performed on YPD agar medium with 200 μM BCS. The donor strains were precultured for 3 d and then the XL280 recipient strain was placed near the donor colonies. The cells were then photographed after being cultured for additional 5 d. Scale bar: 2 mm.
