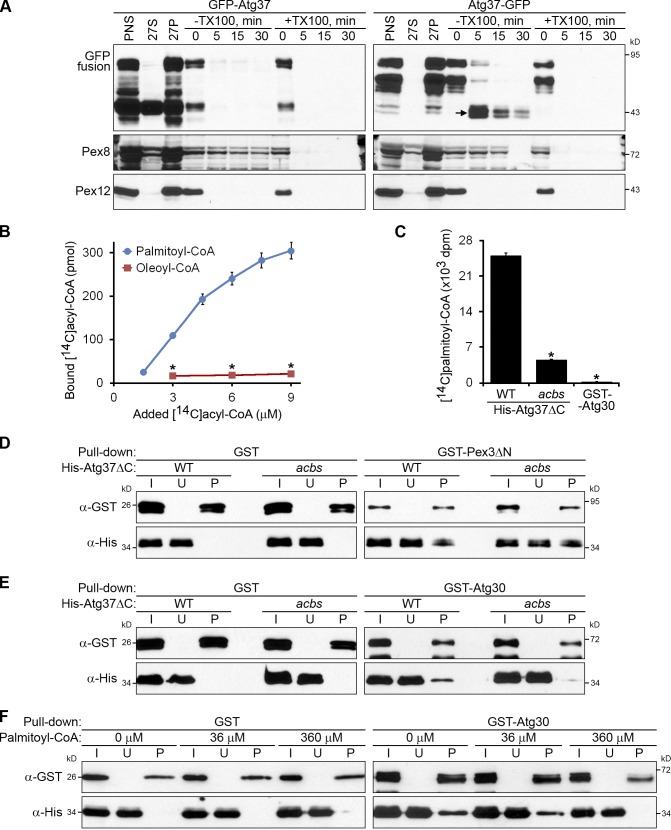
Figure 7.
Atg37 binds palmitoyl-CoA, which competes with Atg30 for binding to Atg37. (A) The acyl-CoA–binding domain of Atg37 faces the cytosol. The organelle pellet, 27P, was subjected to proteinase K and trypsin treatment. TX100, Triton X-100. Arrow points to the protease-protected C-terminal GFP fragment of Atg37-GFP. (B) Atg37ΔC binds specifically to palmitoyl-CoA. The amounts of [14C]palmitoyl-CoA and [14C]oleoyl-CoA bound by 0.07 µM His-Atg37ΔC are shown as the mean ± SD (error bars; n = 3; *, P < 0.0001 between corresponding concentrations). (C) The ACBS of Atg37ΔC is required to bind palmitoyl-CoA. The amounts of [14C]palmitoyl-CoA (9 µM) bound by 0.07 µM His-Atg37ΔC (WT or acbs mutant) or GST-Atg30 are presented as the mean of the disintegration rates ± SD (error bars; n = 3; *, P < 0.001 vs. WT His-Atg37ΔC). (D and E) The acbs mutant of Atg37ΔC can bind Pex3ΔN (D), but not Atg30 (E). (F) Palmitoyl-CoA inhibits the in vitro binding of Atg30 and Atg37ΔC. The 36 µM and 360 µM palmitoyl-CoA partially and completely inhibits the pull-down of 0.28 µM His-Atg37ΔC by 0.14 µM GST-Atg30, respectively. I, input; U, unbound; P, pull-down.