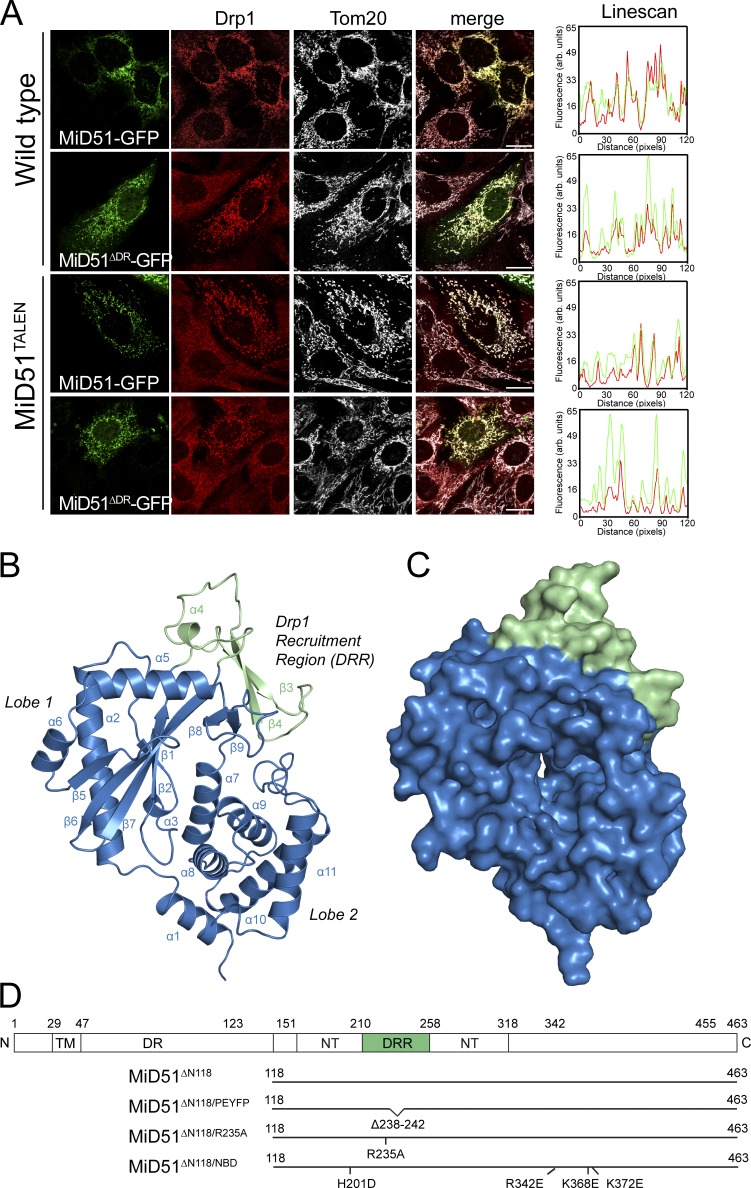
Figure 1.
Crystal structure of a MiD51 cytosolic domain sufficient for Drp1 recruitment. (A) MiD51-GFP and MiD51ΔDR-GFP were expressed in wild-type MEFs and MEFs lacking endogenous MiD51 (MiD51TALEN). Cells were subsequently immunostained for Drp1 and the mitochondrial marker protein Tom20 and visualized by fluorescence microscopy. (right) Linescans demonstrating colocalization of Drp1 with MiD51-GFP and MiD51ΔDR-GFP. Bars, 20 µm. (B) Structure of MiD51ΔN118 displaying the nucleotidyltransferase domain in blue and DRR domain in green. (C) Surface representation of MiD51ΔN118; view and coloring as in B. (D) Topology of MiD51 and illustration of mutant constructs used in this study. TM, transmembrane domain; DR, disordered region; NT, nucleotidyltransferase domain.