Abstract
Cryptosporidium is an obligate intracellular protozoan parasite infecting a wide range of hosts. The current study investigated the genetic profile of Cryptosporidium species in calves in Liverpool, England. Fifty-two calve fecal samples were collected from a farm and initially screened by Auramine Phenol, modified Ziehl-Neelsen and ELISA. PCR analysis of 18S rRNA gene was carried out for the positive samples. Then, positive PCR samples were genotyped by an 18S rRNA- based PCR-RFLP, COWP - based PCR- RFLP; PCR of GP60 and HSP70 genes. Additionally, sequence analysis was carried out based on representative isolates of four loci. Cryptosporidium oocysts and antigens were detected in 34 out of 52 (65.4%) samples using screening techniques. Genotype analysis showed the presence of C. hominis and C. parvum in one and thirteen samples, respectively. Furthermore, subtypes of C. hominis Ib, C. parvum IIa; C. parvum subtype 2 were identified by GP60 and HSP70 sequences, respectively. These findings indicate the diversity of the molecular characteristics of Cryptosporidium species in calves’ isolates. Moreover, referring to the literature; we report two new subtypes of C. parvum IIa and a rare case of C. hominis Ib in calves population.
Key Words: Cryptosporidium, calves, subtype, UK
Cryptosporidium is an obligate intracellular protozoan parasite infecting a wide range of vertebrate hosts such as humans, birds and cattles (1). The genus of Cryptosporidium consists of several genetically distinct species which are morphologically identical. There are some diagnostic methods identifying the parasite phenotypically (2). The classification methods based on parasite phenotype have limitations to distinguish the various species and genotypes found in humans and animals. Molecular genetic techniques are suitable tools to distinguish the different species and genotypes of the parasite. These methods have shown that some parasites are very host-specific while others have a wide host range. For instance, Cryptosporidium parvum is the most prevalent species in cattle and also is the main cause of zoonotic cryptosporidiosis in humans. It has been also found in several hosts including lamb, sheep, goat and so on (3-4).
One of the genotyping tools which are frequently used in the molecular study of this parasite are PCR and PCR- RFLP of the 18S rRNA gene. This gene is highly polymorphic within the genus and is useful as a target for the identification and differentiation of Cryptospori-dium species and genotypes (5-6). Another molecular tool is PCR and PCR- RFLP of the COWP gene which is a single copy gene encoding a major constituent of the inner layer of the Cryptosporidium oocysts wall protein (7). Moreover, PCR and sequence analysis of the 60 kDa glycoprotein (GP60) gene has been frequently used for sub-typing of various Cryptosporidium isolates (8). The GP60 locus has the highest resolution as a single marker for sub-typing of C. parvum isolates because of the existence of nearly one-hundred GP60 sub-genotypes of C. hominis and C. parvum. But this tool does not clearly divide C. hominis and C. parvum into two separate groups (9). Furthermore, heat shock protein 70 kDa (HSP70) gene is a good target for sub-typing and multi-locus study of Cryptosporidium isolates. This gene has a high level of heterogeneity spread over the entire sequence of a variety of Cryptosporidium isolates from human and animal hosts (10).
This approach was conducted to perform a multi-locus study for detection of Cryptosporidium species isolated from calves population using PCR, PCR- RFLP and sequence analysis of the 18S rRNA, COWP, GP60 and HSP70 gene fragments.
Materials and Methods
Sample collection and screening methods
Fifty-two fecal samples of calves with or without diarrhoea which were collected from Liverpool, Northwest England by Professor C.A. Hart, University of Liverpool, in 2003. The samples were stored at 4ºC. The fecal samples were stained by Auramine Phenol (AP) and modified Ziehl-Neelsen (MZN) methods for Cryptosporidium oocysts screening according to methods previously described by Casemore et al. (1985) (11). The ProSpecT ELISA kit (Alexon-Trend, Ramsey, USA) was used for detection of Cryptosporidium Specific Antigen (CSA) based on the manufacturer’s instruction.
Extraction and purification of the parasite DNA
A pea size of sample (about 200 mg) was suspended in 500 μl of ASL buffer (a stool lysis buffer) and then vortexed for 30 seconds. Oocysts were ruptured by subjecting them to a freeze- thaw cycle of + 80ºC for 15 minutes and – 80ºC for 30 minutes. DNA was extracted from the samples using the QIAamp® DNA stool mini kit (QIAGEN Ltd., Crawley, West Sussex, UK). The DNA was further purified according to the kit instruction and stored at -20°C until it was used for PCR assays.
PCR analysis of the 18S rRNA, COWP, GP60 and HSP70 genes
The existence of the Cryptosporidium DNA in positive fecal sample either with microscopy or ELISA was verified by amplification of 18S rRNA gene fragment which produces a product of approximately 840 bp by a nested PCR. The method was performed as previously described by Xiao et al. 1999 (12-13). All DNA extracts positive at 18S rRNA gene locus were further investigated by amplification of the COWP, GP60 and HSP70 gene fragments using primers and conditions previously described by Spano et al. (1997), Zhou et al. (2003) and Sulaiman et al.(2001), respectively (7, 14-15). The primary and secondary primers used in the nested and un-nested PCR analysis of these genes, the annealing temperatures used, and sizes of the expected PCR products are listed in Table 1.
Table 1.
Primers used in the multilocus sequence typing, nature of genetic diversity, and expected sizes of the PCR products
| Locus | Primers | Sequences (5' to 3') | Fragment sites(bp)a | Annealing Temperat-ure(°C) |
References |
|---|---|---|---|---|---|
| 18S rRNA | AL1687 | TTC TAG AGC TAA TAC ATG CG | 156-175 | 55 | Xiao et al.,1999 |
| AL1691 | CCC TAA TCC TTC GAA ACA GGA | 1455-1475 | |||
| AL3032 | GGA AGG GTT GTA TTT ATT AGA TAA AG | 193-218 | 55 | ||
| AL1598 | AAG GAG TAA GGA ACA ACC TCC A | 1008-1029 | |||
| COWP | Cry- 15 | GTA GAT AAT GGA AGA GAT TGT G | 921-943 | 52 | Spano et al., 1997 |
| Cry-9 | GGA CTG AAA TAC AGG CAT TAT CTT G | 1445-1470 | |||
| GP60 | LX001 F1 | ATA GTC TCG CTG TAT TC | 4-21 | 50 | Zhou et al., 2003 |
| LX002 R1 | GCA GAG GAA CCA GCA TC | 906-922 | |||
| LX003 F2 | TCC GCT GTA TTC TCA GCC | 9-27 | 50 | ||
| LX004 R2 | GAG ATA TAT CTT GGT GCG | 480-497 | |||
| HSP70 | F1 | ACT CTA TGA AGG TAT TGA TT | 922-941 | 55 | Sulaiman et al., 2001 |
| R1 | TTA GTC GAC CTC TTC AAC AGT TGG | 2074-2051 | |||
| F2 | CAG TTG CCA TCA GTA GAG | 945-962 | 50 | ||
| R2 | CAA CAG TTG GAC CAT TAG ATC C | 2060-2039 | |||
| INT | GGA CGA GTT TGA ACA TCA A | 1831-1849 |
Expected PCR product size for both Cryptosporidium hominis and C. parvum
All reactions were carried out in a Biometra thermocycler in a Techne Thermal cycler (Techne Ltd, Cambridge, UK). Positive and negative controls were added in every PCR reaction. PCR products were analyzed in 2% agarose gel stained with ethidium bromide. All primers were synthesized by Genosys Oligonucleotides (Sigma Genosys Ltd, UK).
RFLP analysis of the 18S rRNA and COWP genes fragments
RFLP analyses of the 18S rRNA gene fragments were performed using SspI and VspI restriction enzymes (Roche, Germany) for species identification and genotyping of Cryptosporidium species (12-13). Briefly, the restriction digestion was carried out at 37°C for 80 minutes. Each reaction mixture contained 15 μl of the secondary product, 1 μl of SspI (20 U), 2.5 μl of enzyme buffer and 11.5 μl of HPLC water to make a final volume of 30 μl for species identification. The VspI restriction enzyme was used at the same concentration described for SspI. RFLP analysis of the COWP gene fragment was carried out by RsaI endonuclease (Roche, Germany). The restriction digestion was performed at 37°C for 4 hours in a reaction mixture according to the manufacturer’s instruction. The digestion products were analyzed in a 2% and 3.2% agarose gel stained with ethidium bromide for 18S rRNA and COWP genes fragments, respectively. Isolates were assembled according to their RFLP patterns, and a representative of each group was selected for sequence analysis.
DNA sequences analyzing
The PCR products of four genes targets were directly sequenced and were rubbed out from the agarose gel and purified by MicroSpin Columns Kit according to the manufacturer’s instruction (Amersham Biosciences, Buckinghamshire, UK). The Nucleotide sequences were read by the ChromasPro programme (www.technelysium). The consensus sequences and multiple alignments of the DNA sequences were edited using a nucleotide editor program (DNASTAR version 5.06, 2003). Nucleotide sequences obtained from various Cryptosporidium isolates were aligned with published sequences from GenBank by using National Center for Biotechnology Information (NCBI-BLAST program) (http://blast.ncbi.nlm.-nih.gov/Blast.cgi).
The phylogeny of the GP60 gene
The phylogenetic relationships between the GP60 sequences of the Cryptosporidium isolates were assessed with a NJ-tree method using the phylogenetic analysis software Phylogeny (16). The tree was anchored by using C. meleagridis as the out-group as this species showed less similarity to the other species.
Nucleotide sequences accession numbers
Nine sequence PCR samples used in this study have been deposited in the GenBank database under accession no: JX547009,KF533078-79, KF537685-89 and KF577776.
Results
Screening Methods
The prevalence rate of Cryptosporidium infection was obtained 65.4% (34/52) by screening methods. 51.2% (27/52) of the samples were positive by the AP staining method. Twenty five (48.1%) and sixteen (30.8%) out of fifty two sam-ples were positive by modified MZN and the Pro-SpecT ELISA techniques, respectively (Table 2).
Table 2.
Prevalence of Cryptosporidium oocyst in calve fecal samples obtained by screening and molecular techniques
| Methods | Positive N % |
Negative N % |
Total N % |
|---|---|---|---|
| AP | 27 51.2 | 25 48.1 | 52 100 |
| MZN | 25 48.1 | 27 51.2 | 52 100 |
| ELISA | 16 30.8 | 36 79.2 | 52 100 |
| PCR of 18Sr RNA | 14 41.2 | 20 58.8 | 34 100 |
| PCR of COWP | 10 71.4 | 4 28.6 | 14 100 |
| PCR of GP60 | 8 57.1 | 6 42.9 | 14 100 |
| PCR of HSP70 | 8 57.1 | 6 42.9 | 14 100 |
Molecular analysis of 18S rRNA gene
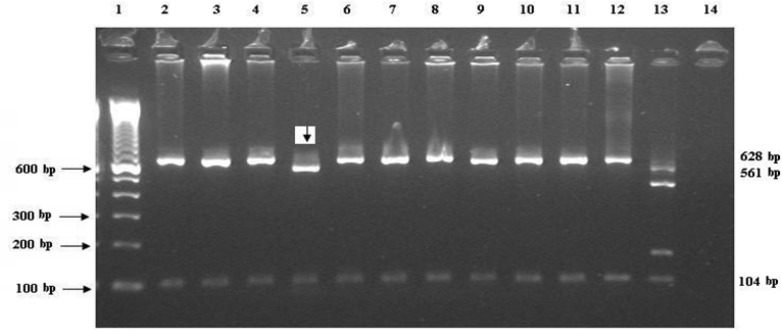
The 18S rRNA gene fragment was amplified in 14 out of 34 positive samples (Table 2). C. parvum was identified in thirteen DNA samples and one isolate (J6) was C. hominis (Figure 1). Five out of fourteen DNA samples were sequenced. Sequence analysis revealed that the C. hominis identified by PCR - RFLP of the 18S rRNA gene had 100% homology to published sequence of C. hominis (accession no. AF481962). Interestingly, one isolate (3H2) which was identified as C. parvum using PCR- RFLP method, showed 100% sequence identity to the published strain of C. hominis with the above accession number. Three other sequenced isolates (17D2, D24 & Calf 44) were 99% -100% similar to the published sequence of C. parvum strain (accession number AY204238).
Fig 1.
Shows the RFLP for the 18S rRNA gene by restriction endonuclease digestion patterns with VspI. Lanes are: 1, Marker (1Kb); 2-4 and 6-12, C. Parvum (628&104 bp bands); 5, C. hominis (561&104 bp bands); 13, Positive control; and 14, Negative control. The samples are from the calf collection, Liverpool, UK
Molecular analysis of COWP gene
The successful amplification of Cryptospo-ridium spp. was observed for 10 out of 14 DNA samples based on COWP gene PCR amplification results (Table 2). PCR- RFLP of the COWP gene revealed seven isolates as C. parvum. In addition, abnormal bands pattern around 200 and 500 bp were observed for two samples (3H2 and 17 D2) and one sample (J6) failed by RFLP of this gene.
Of 5 sequences, two isolates (D24 & Calf44) showed 100% homology with C. parvum (accession number AF266273) and three samples including the isolates identified as C. hominis could not be assembled.
Molecular analysis and the phylogeny of GP60 gene
Among fourteen samples positive for the 18S rRNA gene, 8 (57.1%) isolates yielded a PCR product for the GP60 locus (Table 2). Of the four sequences, three isolates (17D2, D24 & Calf44) were classified as C. parvum IIa allele group (accession no GQ983359 and JF727795). Interestingly, one isolate (3H2) exhibited C. hominis Ib allele group with 100% sequence identity with previously published sequence (accession no JF727788). Another isolate (J6) which was successfully amplified and sequenced for the 18S rRNA gene as C. hominis, did not yield any PCR product for sequencing of the GP60 loci.
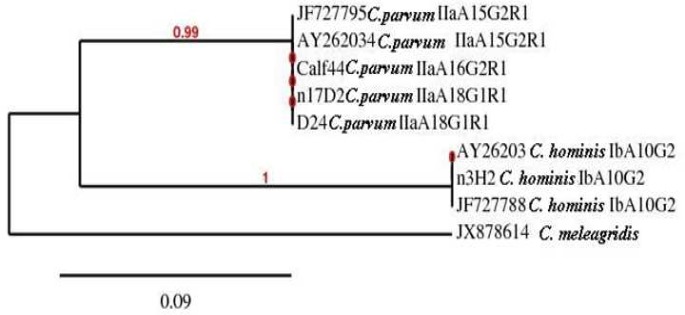
Phylogenetic analysis of the GP60 gene using of the NJ-tree method showed that C. hominis and C. parvum isolates formed two different clades (Figure 2). The phylogenetic position of C. hominis GP60 subtype was consistent with its preliminary classification e.g., subtype Ib isolate (3H2) from the current study grouped with two published Ib sequences (accession numbers AY262031 and JF727788). Furthermore, three isolates of C. parvum IIa grouped with two published IIa sequences (accession numbers JF727795 and AY262034) forming monophyletic clades with maximum nodal support (pp=0.99).
Fig 2.
Phylogeny of Cryptosporidium isolates by a rooted NJ-tree based on GP60 gene. The numbers on branches are bootstrap values greater than 70% and the scale bar indicates an evolutionary distance of 0.09 nucleotides per position in the sequence. The reference sequences accession numbers are inserted
Molecular analysis of HSP70 gene
Of 14 positive samples obtained by the 18S rRNA gene, 8 (57.1%) isolates yielded a PCR product for the HSP70 gene (Table 2). Upon sequence analysis, all (four isolates) were identified as C. parvum subtype 2 which had 100% similarity with the published isolate with accession number KC823128. The interesting aspect of this result was that isolate 3H2 which was identified as C. hominis by sequence analysis of 18S rRNA and GP60 genes; was designated as C. parvum.
Discussion
There are several techniques for detection of Cryptosporidium infection in animal and human fecal samples. Generally, microscopy methods including MZN and AP and also immunoassay techniques are used to detect the parasite's oocysts and antigen (17-19). In the present study, three screening tests were used and the highest prevalence rate of Cryptosporidium infection (51.2%) was obtained with the AP staining method (Table 2). According to the published data, these techniques are fast and sensitive but are not able to distinguish Cryptosporidium species (20). The PCR analysis of 18S rRNA gene showed that 14 (41.2%) samples were positive. The presence of PCR inhibitors in fecal samples (21), the relatively low oocysts count in some of the samples (22), the extraction procedures (23), failure of cell lysis, nucleic acid degradation and capture of an insufficient amount of DNA (24) could be possible explanations for failure of yielding PCR products. These would be possible causes of unsuccessful amplification of PCR of other genes used in this study.
The proportion of C. parvum (92.9%) found in the current study by the PCR- RFLP of 18S rRNA gene was in agreement with those found in other studies (25, 27). For instance, in the UK, 93% of un-weaned calves shed oocysts of C. parvum (25). In Northern Ireland and in the USA, 95% and 85% of low age calves were infected with C. parvum (26-27). Interestingly, one (7.1%) C. hominis Ib subtype was found in the current study which suggested that calves may play a role in the transmission of this to humans. Based on our best knowledge, there are few records of C. hominis in calves and sheeps through the world and therefore the role of these animals in transmission of this species to humans is probably minimal (28). The first natural C. hominis infection in cattle was reported by Smith et al. in 2005 (29) and the second case was reported by Kang'ethe et al., which found that 4% of dairy-keeping households in an urban area in Kenya shed C. hominis oocysts (30). This speeies was also recorded in a goat and in a sheep in the UK (31).
The sequence analysis of the GP60 gene in this study showed that there are three C. parvum IIa allele group. Among these, one C. parvum IIa A16G2R1 (strain Calf44) and two C. parvum IIaA18G1R1 subgenotypes (strains 17D2 & D24) were identified for the first time on based on our best knowledge. These results are in agreement with other studies demonstrating that C. parvum IIa is a common subtype family in humans in addition to calves (3). Moreover, the isolate (3H2) identified as C. hominis by sequencing of 18S rRNA gene, was detected as C. hominis IbA10G2 subgenotype by sequencing of the GP60 locus. The close relationship between this subgenotype of C. parvum allele groups can be stated as a possible explanation for the presence of C. hominis in our samples (15, 32).
In our study, concordant results were obtained with 18S rRNA, COWP, GP60 and HSP70 genes for the majority of isolates; but some exceptions were demonstrated. The isolate (17D2) was identified as C. parvum by PCR- RFLP of 18S rRNA, GP60 and HSP70 sequences, but the COWP gene sequencing failed which may be due to mixed infections or unknown causes. Furthermore, the isolate (3H2) was detected as C. parvum by RFLP of 18S rRNA gene and sequences analysis of HSP70 gene but determined as C. hominis by sequencing of 18S rRNA and GP60 genes.
In conclusion, the multi-locus fragment analysis used in the present study detects polymorphisms in Cryptosporidium isolates in calves. This is the first record of two new subgenotypes of C. parvum IIa subjected to the GenBank data bases. This study reports C. hominis in calve samples and there is a rare possibility of transmission of this species from calves to humans.
Acknowledgments
We acknowledge to Dr. M. L. Chance, Liverpool School of Tropical Medicine; for his kindness, guidance and advices. This work was funded by the Iranian Ministry of Health and Medical Education.
Conflict of interest
The authors declared no conflicts of interest.
References
- 1.Ericsson CD, Steffen R, Okhuysen PC. Traveler's Diarrhea Due to Intestinal Protozoa. Clin infect Dis. 2001;33:110–4. doi: 10.1086/320894. [DOI] [PubMed] [Google Scholar]
- 2.Xiao L, Fayer R, Ryan U, et al. Cryptosporidium taxonomy: recent advances and implications for public health. Clin Microbiol Rev . 2004;17:72–97. doi: 10.1128/CMR.17.1.72-97.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao L, Feng Y. Zoonotic cryptosporidiosis. FEMS Immunol Med Microbiol. 2008;52:309–23. doi: 10.1111/j.1574-695X.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- 4.Xiao L, Ryan UM. Cryptosporidiosis: an update in molecular epidemiology. Curr Opin Infect Dis . 2004;17:483–90. doi: 10.1097/00001432-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Collins MD, McDonald V, et al. PCR cloning and nucleotide sequence determination of the 18S rRNA genes and internal transcribed spacer 1 of the protozoan parasites Cryptosporidium parvum and Cryptosporidium muris. Biochim Biophys Acta . 1992;1131:317–20. doi: 10.1016/0167-4781(92)90032-u. [DOI] [PubMed] [Google Scholar]
- 6.Xiao L, Escalante L, Yang C, et al. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999;65:1578–83. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spano F, Putignani L, McLauchlin J, et al. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett . 1997;150:209–17. doi: 10.1016/s0378-1097(97)00115-8. [DOI] [PubMed] [Google Scholar]
- 8.Jex AR, Gasser RB. Genetic richness and diversity in Cryptosporidium hominis and C. parvum reveals major knowledge gaps and a need for the application of "next generation" technologies research review. Biotechnol Adv . 2010;28:17–26. doi: 10.1016/j.biotechadv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Xiao L, Bern C, Sulaima IM, et al. Molecular epidemiology of human cryptosporidiosis. Cryptosporidium: from molecule to disease. Amsterdam: Elsevier Inc; 2003. pp. 121–46. [Google Scholar]
- 10.Sulaiman IM, Morgan UM, Thompson RC, et al. Phylogenetic relationships of Cryptosporidium parasites based on the 70-kilodalton heat shock protein (HSP70) gene. Appl Environ Microbiol . 2000;66:2385–91. doi: 10.1128/aem.66.6.2385-2391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casemore DP, Armstrong M, Sands RL. Laboratory diagnosis of cryptosporidiosis. J Clin Pathol . 1985;38:1337–41. doi: 10.1136/jcp.38.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao L, Morgan UM, Limor J, et al. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol. 1999;65:3386–91. doi: 10.1128/aem.65.8.3386-3391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghaffari S, Kalantari N. Molecular analysis of 18S rRNA gene of Cryptosporidium parasites from patients living in Iran, Malawi, Nigeria and Vietnam. Int J Mol Cell Med. 2012;1:153–69. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Singh A, Jiang J, et al. Molecular surveillance of Cryptosporidium spp. in raw wastewater in Milwaukee: implications for understanding outbreak occurrence and transmission dynamics. J Clin Microbiol . 2003;41:5254–7. doi: 10.1128/JCM.41.11.5254-5257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sulaiman IM, Lal AA, Xiao L. A population genetic study of the Cryptosporidium parvum human genotype parasites. J Eukaryot Microbiol . 2001;Suppl:24 S–7S. doi: 10.1111/j.1550-7408.2001.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 16.Dereeper A, Guignon V, Blanc G, et al. Phylogeny. fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res . 2008;36:W465–9. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhnert-Paul Y, Bangoura B, Dittmar K, et al. Cryptosporidiosis: comparison of three diagnostic methods and effects of storage temperature on detectability of cryptosporidia in cattle faeces. Parasitol Res . 2012;111:165–71. doi: 10.1007/s00436-011-2813-6. [DOI] [PubMed] [Google Scholar]
- 18.Moodley D, Jackson TF, Gathiram V, et al. A comparative assessment of commonly employed staining procedures for the diagnosis of cryptosporidiosis. S Afr Med J . 1991;79:314–7. [PubMed] [Google Scholar]
- 19.Cirak VY, Bauer C. Comparison of conventional coprosco-pical methods and commercial coproantigen ELISA kits for the detection of Giardia and Cryptosporidium infections in dogs and cats. Berl Munch Tierarztl Wochenschr . 2004;117:410–3. [PubMed] [Google Scholar]
- 20.Amar CF, Dear PH, McLauchlin J. Detection and identification by real time PCR-RFLP analyses of Cryptosporidium species from human faeces. Lett Appl Microbiol . 2004;38:217–22. doi: 10.1111/j.1472-765x.2004.01473.x. [DOI] [PubMed] [Google Scholar]
- 21.Monteiro L, Bonnemaison D, Vekris A, et al. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J Clin Microbiol . 1997;35:995–8. doi: 10.1128/jcm.35.4.995-998.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLauchlin J, Pedraza-Diaz S, Amar-Hoetzeneder C, et al. Genetic characterization of Cryptosporidium strains from 218 patients with diarrhea diagnosed as having sporadic cryptosporidiosis. J Clin Microbiol. 1999;37:3153–8. doi: 10.1128/jcm.37.10.3153-3158.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson DW, Pieniazek NJ, Griffin DW, et al. Development of a PCR protocol for sensitive detection of Cryptosporidium oocysts in water samples. Appl Environ Microbiol . 1995;61:3849–55. doi: 10.1128/aem.61.11.3849-3855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey CM, Lee H, Trevors JT. Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidiumhominis oocyst. Water Res . 2004;38:818–62. doi: 10.1016/j.watres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Brook EJ, Anthony Hart C, French NP, et al. Molecular epidemiology of Cryptosporidium subtypes in cattle in England. Vet J . 2009;179:378–82. doi: 10.1016/j.tvjl.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Thompson HP, Dooley JS, Kenny J, et al. Genotypes and subtypes of Cryptosporidium spp. in neonatal calves in Northern Ireland. Parasitol Res. 2007;100:619–24. doi: 10.1007/s00436-006-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santin M, Trout JM, Xiao L, et al. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet Parasitol . 2004;122:103–17. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124:80–9. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Smith HV, Nichols RA, Mallon M, et al. Natural Cryptosporidiumhominis infections in Scottish cattle. Vet Rec . 2005;156:710–1. doi: 10.1136/vr.156.22.710. [DOI] [PubMed] [Google Scholar]
- 30.Kang'ethe EK, Mulinge EK, Skilton RA, et al. Cryptosporidium species detected in calves and cattle in Dagoretti, Nairobi, Kenya. Trop Anim Health Prod . 2012;44 (Suppl 1):S25–31. doi: 10.1007/s11250-012-0202-5. [DOI] [PubMed] [Google Scholar]
- 31.Giles M, Chalmers R, Pritchard G, et al. Cryptosporidiumhominis in a goat and a sheep in the UK. Vet Rec. 2009;164:24–5. doi: 10.1136/vr.164.1.24. [DOI] [PubMed] [Google Scholar]
- 32.Peng MM, Matos O, Gatei W, et al. A comparison of Cryptosporidium subgenotypes from several geographic regions. J Eukaryot Microbiol . 2001;(Supp l):28S–31S. doi: 10.1111/j.1550-7408.2001.tb00442.x. [DOI] [PubMed] [Google Scholar]