Abstract
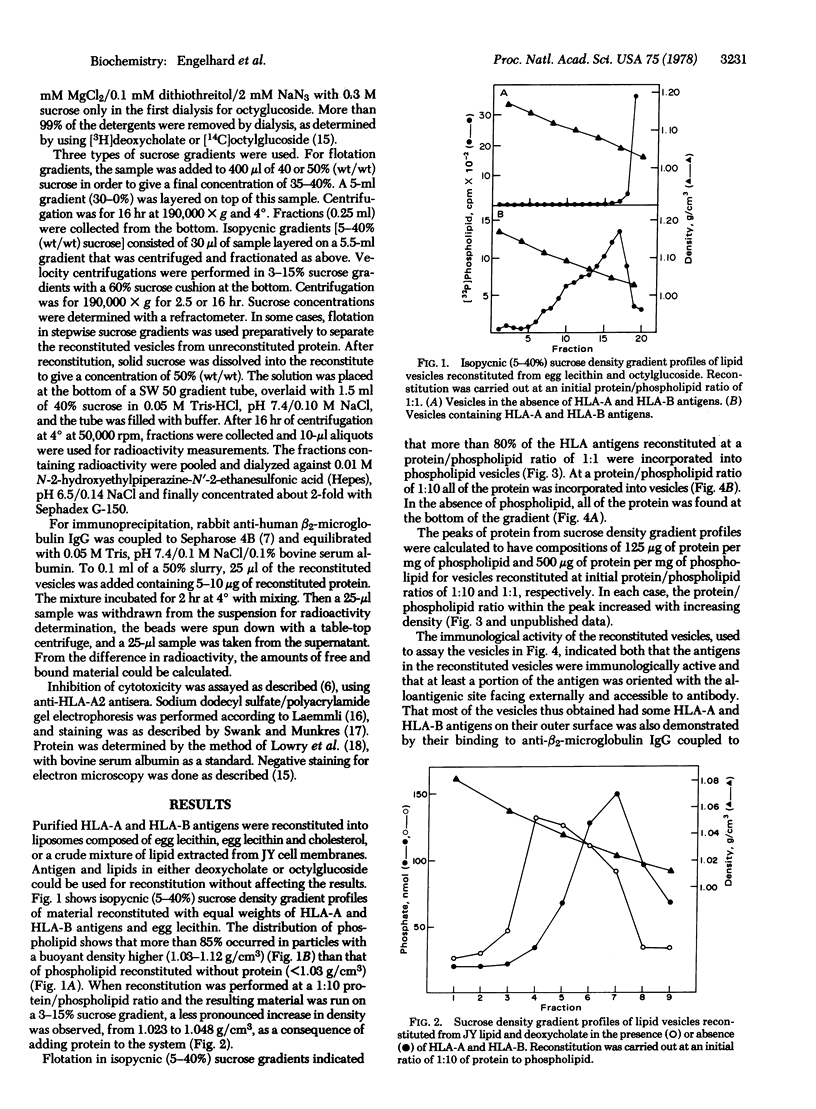
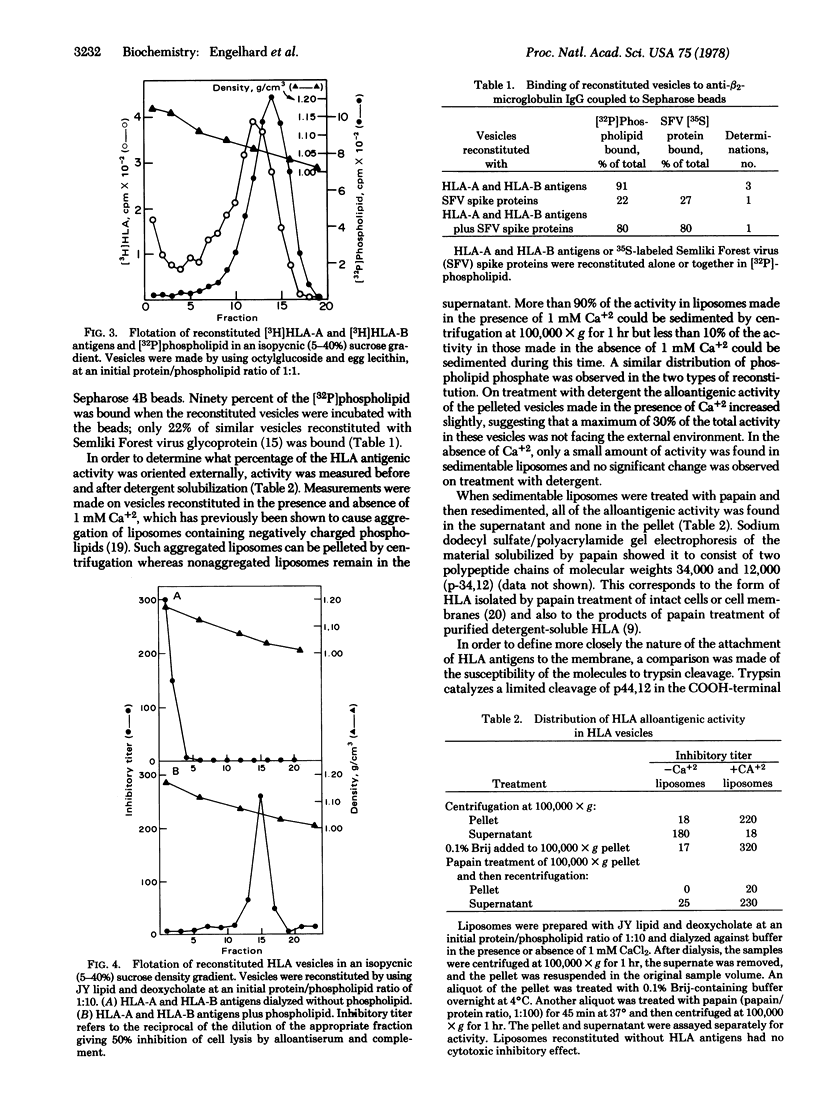
Purified detergent-soluble human histocmpatibility antigens (HLA-A and HLA-B) were reconstituted into phospholipid vesicles by mixing the protein and lipid together in the presence of either octylglucoside (octyl-beta-D-glucopyranoside) or deoxycholate and removing the detergent by dialysis. The resulting preparation consisted of lipid vesicles containing all or most of the added protein. The protein in the vesicles was antigenically active, as demonstrated by specific binding to anti-beta2-microglobulin IgG-Sepharose beads and by specific inhibition of alloantibody and complement-mediated cytotoxicity. Protein incorporated into vesicles at a protein/phospholipid ratio of 1:10 showed an asymmetric distribution of the HLA-A and HLA-B molecules, with virtually all of the antigens oriented facing the external medium. Cleavage experiments with proteases showed that the molecule was attached to the vesicle membrane via the COOH terminus, consistent with its proposed structure in intact cellular plasma membranes. Electron micrographs of the vesicles showed 50-60 A knobs on the outer surface similar to structures observed for other membrane proteins. HLA-A and HLA-B could also be incoporated into vesicles together with Semliki Forest virus membrane proteins. The resulting preparations should be useful in defining the molecular interactions involving HLA-A and HLA-B antigens in the immune response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. J., Schendel D. J., Bach M. L., Bach F. H., Klein J., Stimpfling J. H. Cell-mediated lympholysis. Importance of serologically defined H-2 regions. J Exp Med. 1973 May 1;137(5):1303–1309. doi: 10.1084/jem.137.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. The major histocompatibility complex determines susceptibility to cytotoxic T cells directed against minor histocompatibility antigens. J Exp Med. 1975 Dec 1;142(6):1349–1364. doi: 10.1084/jem.142.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P., Turner M. J., Strominger J. L. Papain-solubilized HL-A antigens from cultured human lymphocytes contain two peptide fragments. Proc Natl Acad Sci U S A. 1973 May;70(5):1603–1607. doi: 10.1073/pnas.70.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curman B., Ostberg L., Peterson P. A. Incorporation of murine MHC antigens into liposomes and their effect in the secondary mixed lymphocyte reaction. Nature. 1978 Apr 6;272(5653):545–547. doi: 10.1038/272545a0. [DOI] [PubMed] [Google Scholar]
- Fast L. D., Fan D. P. Dissociated and reconstituted subcellular alloantigen capable of stimulating mouse cytotoxic T lymphocytes in vitro. J Immunol. 1978 Apr;120(4):1092–1096. [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Fries E., Kartenbeck J. Reconstitution of Semliki forest virus membrane. J Cell Biol. 1977 Dec;75(3):866–880. doi: 10.1083/jcb.75.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mescher M., Sherman L., Lemonnier F., Burakoff S. The induction of secondary cytolytic T lymphocytes by solubilized membrane proteins. J Exp Med. 1978 Mar 1;147(3):946–951. doi: 10.1084/jem.147.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Jacobson K., Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975 Jul 3;394(3):483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Robb R. J., Strominger J. L. Rapid purification of detergent-solubilized HLA antigen by affinity chromatography employing anti-beta2-microglobulin serum. J Biol Chem. 1976 Sep 10;251(17):5427–5428. [PubMed] [Google Scholar]
- Shearer G. M., Rehn T. G., Garbarino C. A. Cell-mediated lympholysis of trinitrophenyl-modified autologous lymphocytes. Effector cell specificity to modified cell surface components controlled by H-2K and H-2D serological regions of the murine major histocompatibility complex. J Exp Med. 1975 Jun 1;141(6):1348–1364. doi: 10.1084/jem.141.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Mann D. L., DeFranco A. L., Strominger J. L. Detergent solubilization, purification, and separation of specificities of HLA antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1977 Jul 10;252(13):4682–4693. [PubMed] [Google Scholar]
- Springer T. A., Robb R. J., Terhorst C., Strominger J. L. Submit and disulfide structure of monomeric and dimeric forms of detergent-soluble HLA antigens. J Biol Chem. 1977 Jul 10;252(13):4694–4700. [PubMed] [Google Scholar]
- Springer T. A., Strominger J. L. Detergent-soluble HLA antigens contain a hydrophilic region at the COOH-terminus and a penultimate hydrophobic region. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2481–2485. doi: 10.1073/pnas.73.7.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Turner M. J., Sanderson A. R. The preparation of liposomes bearing human (HLA) transplantation antigens. Biochem J. 1978 May 1;171(2):505–508. doi: 10.1042/bj1710505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. H-2 compatability requirement for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T-cell specificities are associated with structures coded for in H-2K or H-2D;. J Exp Med. 1975 Jun 1;141(6):1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]