Abstract
Type IV pili (T4P) are hair-like appendages found on the surface of a wide range of bacteria belonging to the β-, γ-, and δ-Proteobacteria, Cyanobacteria and Firmicutes. They constitute an efficient device for a particular type of bacterial surface motility, named twitching, and are involved in several other bacterial activities and functions, including surface adherence, colonization, biofilm formation, genetic material uptake and virulence. Tens of genes are involved in T4P synthesis and regulation, with the majority of them being generally named pil/fim genes. Despite the multiple functionality of T4P and their well-established role in pathogenicity of animal pathogenic bacteria, relatively little attention has been given to the role of T4P in plant pathogenic bacteria. Only in recent years studies have begun to examine with more attention the relevance of these surface appendages for virulence of plant bacterial pathogens. The aim of this review is to summarize the current knowledge about T4P genetic machinery and its role in the interactions between phytopathogenic bacteria and their plant hosts.
Keywords: type IV pili, twitching, virulence, biofilm
1. Introduction
Pili or fimbriae are hair-like appendages found on the surface of many bacteria. There are several types of pili, which differ in their mechanisms of assembly, structure and function. Among them, type IV pili (T4P) are widespread among diverse members of the β-, γ-, and δ-Proteobacteria, Cyanobacteria and Firmicutes. T4P are therefore found in both Gram-negative and Gram-positive bacteria, supporting an ancient origin for this kind of pili [1,2]. T4P are proteinaceous, flexible filaments with a diameter of 5–8 nm, which extend up to several micrometers in length. They are generally located at one or both poles of a cell, where they mediate twitching motility, an efficient and versatile flagellar-independent form of bacterial surface motility [3,4] (supplementary movie 1). T4P have also been shown to be involved in a variety of other bacterial activities and features, including surface adhesion and colonization, biofilm formation, genetic material uptake and virulence [1,5].
Pseudomonas aeruginosa has been the principal model for investigation of genetic and functional aspects of T4P. P. aeruginosa can be easily cultured and molecularly manipulated, and there is a strong basis of genetic and genomic approaches available for this bacterium. In addition, twitching motility can be easily scored in this bacterium by the naked eye. Importantly, P. aeruginosa is an opportunistic pathogen, being the main causal agent of lung damage in cystic fibrosis patients and responsible for severe infections in immunocompromised individuals. It is also able to cause disease in a wide range of animals and plants [4,6,7]. Other well-studied bacterial species in which T4P have been thoroughly investigated are Myxococcus xanthus, Neisseria gonorrhoeae and Neisseria meningitidis. M. xanthus is a model organism for studying bacterial social behavior and development (specifically fruiting body formation), features that are strongly associated with T4P [4,8-10]. The above Neisseria species are important human pathogens and possess a high natural competence for transformation [4,11].
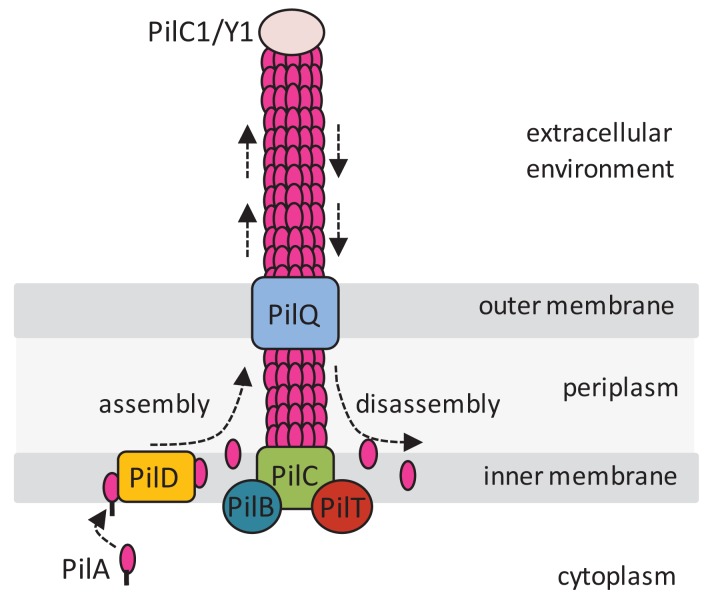
T4P are mainly composed of thousands of copies of a small (13–23 kDa) subunit named pilin (in most cases termed PilA). All type IV pilins are synthesized as prepilins, which are processed by a peptidase that removes their N-terminal leader peptide (Figure 1). Based on differences in the length of their leader peptides and mature sequences, type IV pilins are divided into two subgroups, type IVa and type IVb. Type IVa pilins possess shorter leader peptides (less than 10 aa) and a typical length of about 150-160 aa. In contrast, type IVb pilins exhibit longer leader peptides (about 15–30 aa), and are either longer (180-200 aa) or shorter (about 40–50 aa) than type IVa pilins [5]. Interestingly, while type IVa pilins are present in a broad range of bacteria, type IVb pilins are found almost exclusively in enteric pathogens like Vibrio cholerae, enteropathogenic and enterotoxigenic Escherichia coli and Salmonella enterica [12].
Figure 1.
Type IV pili (T4P) structure and function. The T4P filament is mainly composed of pilin (PilA) subunits that are synthesized as prepilin and cleaved by the action of PilD, which also methylates N-terminal phenylalanine of the mature pilin. PilA units are assembled into the pilus by the cytoplasmic membrane protein PilC, with the filament emerging out via the outer membrane secretin PilQ. The ATPase proteins PilB and PilT mediate pilus assembly (extension) and disassembly (retraction), respectively. Proteins are named according to the Pseudomonas aeruginosa nomenclature, which is the one generally used for plant pathogenic bacteria (see text and Table 1 for functions of other proteins and alternative nomenclatures). This figure was prepared based on a figure from Chen and Dubnau [13].
The secondary structure of pilin, as well as its structure in the pilus filament, was first reported in 1995 for N. gonorrhoeae by Parge and colleagues [14]. Despite a limited sequence similarity beyond the first 25 aa residues, type IV pilins share a common architecture, being mainly characterized by a highly hydrophobic N-terminal region of ∼60 residues arranged in α-helices and a more variable and hydrophilic C-terminal region, mainly organized as 4 or 5 anti-parallel stranded-β-sheets. The N-terminal regions of adjacent monomers form an α-helical coiled-coil structure that is tucked inside the pilus fiber and covered by the C-terminal regions of adjacent monomers, which form a scaffold of β-strands. Although type IVa and type IVb pilins share a similar architecture, they differ in their β-sheet topology [2]. While most genes involved in biogenesis and regulation of type IVa pili are named pil genes, the gene nomenclature for type IVb pili is not conserved among different bacteria (Table 1).
Table 1.
Core components of T4P structure and function according to the Pseudomonas aeruginosa nomenclature. Some of the alternative names of homologous proteins in other bacteria are also indicated.
| Protein | Function | Alternative names a |
|---|---|---|
| PilA | Pilin, main component of the T4P filament | PilE b, FimA c |
| PilB | ATPase that mediates pilus assembly and extension | PilF b, FimN c |
| PilC | Inner membrane protein | PilG b, FimO c |
| PilD | Prepilin leader peptidase/pilin N-terminus methylase | FimP c |
| PilQ | Outer membrane secretin | ComE d |
| PilT | ATPase that mediates pilus retraction and disassembly | PilU e |
Several alternative names are shown for components of type IVa pili. The nomenclature of type IVb proteins is not conserved and is not indicated here. For such information, see the review of Craig and Li [12];
Neisseria spp.;
Dichelobacter nodosus;
Haemophilus influenzae;
Pseudomonas aeruginosa.
In the following sections, we briefly describe the bacterial functions and activities that have been associated with T4P and the genetic machinery involved in its biogenesis and regulation. Next, we summarize the current knowledge of the role played by T4P in virulence and fitness of plant pathogenic bacteria. The structure of the T4P apparatus, as well as the complex mechanisms by which T4P assemble and disassemble, were reviewed recently [1,2,12,15] and will therefore not be addressed in detail in this review.
2. Features Associated with T4P
2.1. Twitching Motility
The term “twitching motility” was first introduced by Lautrop in 1961 to describe a type of flagellar-independent bacterial surface motility [16]. However, it was in the early 1970s that the association between twitching motility and T4P started to be elucidated by Henrichsen and colleagues. They showed that in Moraxella spp., Acinetobacter spp. and other Gram-negative strains, twitching motility strongly correlates with the formation of polar fimbriae, which later were termed type IV pili/fimbriae [17-19]. Henrichsen studied twitching motility on moist solid media and defined twitching motility as a type of surface translocation that produces spreading zones at the colony edges [20]. These twitching zones can be formed by one or several layers of cells, depending on the bacterial strain and growth conditions. Similar findings on the association between surface motility and pili were reported in 1976 by MacRae and McCurdy for M. xanthus [21], in which this type of motility is referred to as “gliding”.
Microscopic studies with non-piliated and hyperpiliated mutants of P. aeruginosa by Bradley in 1980 confirmed that T4P are required for twitching motility and evidenced that fimbrial extension and retraction is the mechanical basis of this movement [22]. Essentially, the distal end of T4P adheres to the surface and then retraction from the base of the pilus provides the force that pulls the cell forward. Eight years earlier, Bradley provided the first evidence of the role of T4P retraction in phage invasion. He showed that bacteriophage PP7 attaches to the tip of the P. aeruginosa pilus, and the average length of the pilus is significantly reduced upon incubation of the bacterium with the phage, bringing the phage into contact with the bacterial cell surface [23,24].
Strong evidence for pilus retraction was reported in 2000 for N. gonorrhoeae T4P by Merz and colleagues, who measured the velocity, timing and force of pilus retraction [25]. The retraction velocity was about 1.2 μm s−1, a value that correlated with the observed cell motility rates (about 1 μm s−1), supporting the idea that pilus retraction is responsible for twitching motility in this bacterium. Observations consistent with the association between T4P retraction and cell movement were reported in the same year for M. xanthus in studies comparing wild type and T4P mutants of this bacterium [26]. These findings were further supported by studies with P. aeruginosa by Skerker and Berg, who used a fluorescent dye to label T4P, thus allowing microscopic visualization of pili in live bacteria [27]. This study revealed that, similar to what was inferred for N. gonorrhoeae and M. xanthus, T4P in P. aeruginosa retract, and pilus retraction is responsible for pulling the cell forward. Here too, the velocity of pilus retraction correlated with that of cell movement (∼0.5 and ∼0.3 μm s−1, respectively). In addition, it was shown that pilus extension is not associated with cell movement, indicating that T4P can pull but not push. The mechanism by which T4P generate twitching motility was reviewed recently by Burrows [15].
2.2. Adhesive Properties and Biofilm Formation
No matter the lifestyle of a given bacterium, the ability to adhere to surfaces and to other cells is a crucial property, as it allows bacteria to efficiently colonize different niches, often through formation of biofilms. Biofilms are the most common mode of bacterial growth in nature and can be defined as communities of microorganisms attached to a surface [28]. Substantial evidence has accumulated since the mid-1980s on the adhesive properties of T4P and their importance for biofilm formation in P. aeruginosa and other bacteria [28-34]. Indeed, O'Toole and Kolter [35] identified several pil− mutants of P. aeruginosa in a screen for defective biofilm formation ability. The pil− mutants were shown to poorly attach to a plastic surface relative to the wild type, and they were unable to move and form cell aggregates. In agreement with these results, Heydorn and colleagues showed that P. aeruginosa pil− mutants were severely affected in biofilm formation in a flow chamber relative to wild-type cells [36].
T4P functionality, particularly pilus retraction, is generally important for the adhesive properties conferred by these filaments. T4P retraction is necessary, not only for twitching motility, but also for formation of microcolonies in N. gonorrhoeae [25]. T4P retraction requires a protein that possesses ATPase activity, generally named PilT. pilT mutants are able to produce T4P; however, these mutants are often hyperpiliated [33,37] and lack pilus retraction and twitching abilities. In N. gonorrhoeae and M. xanthus, pilT mutants are able to tether to inert surfaces and to other cells, but they are unable to form microcolonies or a wide raft of cells, respectively [25,38]. In P. aeruginosa, under static conditions, pilT mutants form a much denser biofilm than the wild type; however, under flow conditions, these mutants produce biofilm mushroom-like structures that are less dense than those produced by the wild type [33]. A pilA mutant of this bacterium, impaired in pilin synthesis, was severely affected in biofilm formation under static conditions and was not able to form biofilms at all under flow conditions [33]. In agreement with these results, Klausen and colleagues showed that, in P. aeruginosa, twitching motility is required for the development of biofilm mushroom-like caps [39]. Similarly, in M. xanthus, T4P-mediated motility was shown to be required for normal fruiting body formation [10]. Recently, Conrad and colleagues showed that in P. aeruginosa, T4P interact cooperatively with flagella to influence the biofilm morphology through changes in movement orientation [40].
2.3. Virulence
The ability to adhere to the host cell surface is an important requisite for pathogenicity, as this step is required to initiate an infection. Several studies with various animal pathogenic bacteria have shown that bacteria that have lost T4P possess reduced adhesion and host colonization abilities, which are often associated with reduced virulence or lack of pathogenicity depending on the system investigated [29-32,41-43]. In many Gram-negative pathogenic bacteria, type III secretion systems (T3SS) play an important role in virulence by injecting effector proteins into eukaryotic host cells where they promote disease [44,45]. In P. aeruginosa, expression of T3SS-related genes was shown to be induced by bacterial contact to host cells mediated by T4P [46]. Moreover, global regulatory systems are known to promote virulence in this bacterium through mediation of toxin production, biosynthesis of flagella and T4P, and type III secretion [47,48]. Recently it has been shown that common two-component regulatory systems regulate the expression of T4P and multidrug resistance-related genes, revealing an intricate regulatory network for pili formation and antibiotic resistance [49]. Importantly, T4P are able to elicit host immune responses, and vaccines produced with purified pili can provide protection against infection with serologically related strains [4,50,51].
For several pathogenic bacteria, the contribution of T4P to virulence depends not only on the presence of T4P but also on their retraction ability. pilT mutants of P. aeruginosa and N. gonorrhoeae as well as bfpF (pilT homolog) of enteropathogenic E. coli display a significant reduction (or even lack) of cytotoxicity [43,52-54]. As mentioned, pilT mutants generally produce T4P but lack pilus retraction and twitching ability. As detailed in section 4 of this review, functional T4P are also required for wild-type levels of virulence in several plant pathogenic bacteria.
2.4. DNA Uptake and Protein Secretion
Several genes required for T4P assembly are homologous to genes involved in type II protein secretion and DNA uptake systems, indicating that these systems share a common evolutionary origin and architecture [4,55]. Homologous genes in these systems encode several components including pilins and pseudopilins, prepilin-processing leader peptidases, ATPases, multi-spanning transmembrane proteins and outer-membrane secretins [7,55]. Interestingly, in P. aeruginosa, PilA is required for optimal protein secretion [56]. T4P are strongly associated with competence for DNA transformation in many bacteria, thus this type of pilus is thought to play an important role in horizontal gene transfer [4,55]. Due to their high natural competence for DNA transformation, Neisseria species have been the principal model organisms for investigation of the association between transformation competence and T4P [11,57].
The mechanism by which T4P mediate DNA transformation has not been elucidated. Nevertheless, experimental evidence from several studies indicates that some of the proteins involved in pilus assembly and disassembly systems, but not the pilus structures per se, are essential for DNA translocation [55]. In support of this notion, the space formed within the core of the T4P filament is not sufficiently wide to allow nucleic acid passage [4]. Moreover, several naturally transformable bacteria, such as Bacillus subtilis, Streptococcus pneumoniae and Haemophilus influenzae do not produce T4P, although their transformation machineries comprise components that are similar to pilins and are required for transformation competence [55].
3. Genes Involved in T4P Synthesis and Variability of Pilin
3.1. Core Components of the T4P Machinery
Several recent reviews have described the current knowledge about genes involved in T4P synthesis and regulation [3,4,12,15]; therefore, these aspects will not be addressed in detail in this review. In P. aeruginosa, over 40 genes have been shown to be involved in T4P assembly, function and regulation [4,7,58]. The biogenesis and function of T4P are controlled by multiple signal transduction systems, including two-component regulatory systems [59-61], global carbon metabolism regulators [62], chemosensory systems [26,63-67] and quorum sensing [68,69]. In many bacterial species, the core genes involved in T4P assembly are located in the same operon and encode key conserved components (Table 1) that have homologs in type II secretion and archaeal flagellar systems [5,70-72]. For consistency and clarity, here we use the terminology used in P. aeruginosa, which is the one adopted for most plant pathogenic bacteria.
Some of the key protein components necessary for T4P synthesis and function, known to be conserved among bacterial species, are shown in Figure 1. These include: (i) PilA, the pilin subunit that constitutes the main structural component of T4P; (ii) PilD, a peptidase/N-methylase situated in the inner membrane of the bacterial cell. PilD recognizes the conserved N-terminal region of PilA, cleaves the leader peptide of prepilin proteins in a step that is necessary for T4P assembly [73]. PilD also catalyzes N-methylation of the amino-terminal phenylalanine of mature pilin [74], and cleaves other prepilin-like leader sequences of proteins involved in secretion [73]; (iii) PilB and PilT proteins, which are necessary for filament extension/polymerization and retraction, respectively [1,75]. Both PilB and PilT are ATPases that function as the motor responsible for twitching motility and are located in the cell inner membrane, or periplasm (PilB) and cytoplasm (PilT) [1]. PilT mutants are generally hyperpiliated, non-motile [76], and resistant to pilus-specific bacteriophages [77]. Mutations in PilB abolish production of T4P [76]. PilB and PilT are very well conserved among bacteria [78]; (iv) PilQ, an outer membrane secretin that is involved in export of pilin subunits [79]. Mutagenesis analyses in pilQ of N. gonorrhoeae indicate that this protein may have multiple roles related to pilus extrusion and function and host adherence [79].
3.2. Variability in Pilin Sequence
Pilin (PilA) is a logical choice for studies of diversity, since it is the main structural subunit of T4P. As mentioned, besides the highly conserved first 25 aa residues of the N-terminus, PilA proteins show limited sequence similarity [12]. Nevertheless, PilA proteins share a conserved structure among bacteria. They are small proteins of 13–23 kDa, consisting of a hydrophobic N-terminal region (∼60 residues) arranged in α-helices and a hydrophilic C-terminal region, mainly organized as β-sheets [2,14,15]. At the C-terminus, PilA has two cysteine residues that create a conserved disulfide-bonded loop (DSL) of variable size between 12 and 31 amino acids [80]. The DSL is believed to be exposed at the pili surface where it interacts with eukaryotic glycolipid receptors [80] and is proposed to have a role in adherence. Amino acids inside the DSL have key roles in T4P assembly and twitching motility [81].
Pilin sequences in Neisseria species (termed PilE) undergo great antigenic variation, although they were recently shown to be conserved among specific clonal complexes of N. meningitidis [82]. Due to sequence variation, pilin has been discarded as an optional vaccine target for Neisseria spp. Studies on population diversity among isolates of P. aeruginosa [80], defined five phylogenetic groups based on T4P sequences, particularly focusing on the pilin gene. It was found that one group was more prevalent in cystic fibrosis patients and environmental samples, as opposed to other types of clinical samples. This work showed a strong relationship between a specific pilin allele in P. aeruginosa and cystic fibrosis patients, indicating a possible role of T4P in host specificity. Moreover, a specific pilA variant was associated with isolates causing keratitis [83], further supporting the role of this gene in disease development. In another study, pilA was used to divide the human pathogen Moraxella catharralis into two clades based mainly on DNA sequence divergence at the C-terminus [84]. Isolates from both clades did not differ in pathogenicity despite the diverse origins of isolation from different patients and geographic locations [84]. The authors concluded that, for this bacterium, this highly conserved gene is a good candidate as an antigen for vaccine development.
Few studies have dealt with variability of gene sequences and structures of pil genes other than the pilin-encoding pilA. Among the few exceptions are recent studies of N. gonorrhoeae pilQ divergence among clinical isolates. The authors found nine groups differing in amino acid sequence among the tested population, but could not find a correlation with differences in antibiotic resistance [85].
3.3. Pilin Sequence Variability among Plant Pathogenic Bacteria
Some studies on sequence variability of pilin genes have been performed with animal pathogens, and these are primarily restricted to studies of pathogens within the same genus or species. To our knowledge, there are no previous studies addressing the variability of pilin (pilA) genes among plant pathogenic bacteria or comparing pilin gene sequences from plant and animal pathogenic bacteria. For this review, we conducted phylogenetic analyses of 71 pilin gene sequences (usually annotated as pilA) from plant and mammalian bacterial pathogen sequences available in GenBank (Table S1, supplementary material). Gene sequences were acquired only from complete genome sequences of well-known Gram-negative bacterial pathogens with satisfactory annotation, and the pilin gene designation was confirmed by BLAST. For alleles present in multiple isolates, the pilin gene of only one representative isolate was included. The ClustalW [86] plugin in Geneious v5.4 [87] was used to align nucleotide sequences. Bayesian phylogenetic (BP) analyses were conducted using MrBayes v3.1.2 [88] with 2 chains of 2 million generations each using the GTR + I + Γ model. Trees were sampled every 200 generations, and the first 5,000 trees (10%) were discarded as burnin for each chain prior to generating the extended majority rule consensus tree. Maximum likelihood (ML) analyses were conducted using RAxML v7.0.3 [89] using the rapid BS algorithm [90] with 1,000 bootstraps and the GTR + I + Γ model.
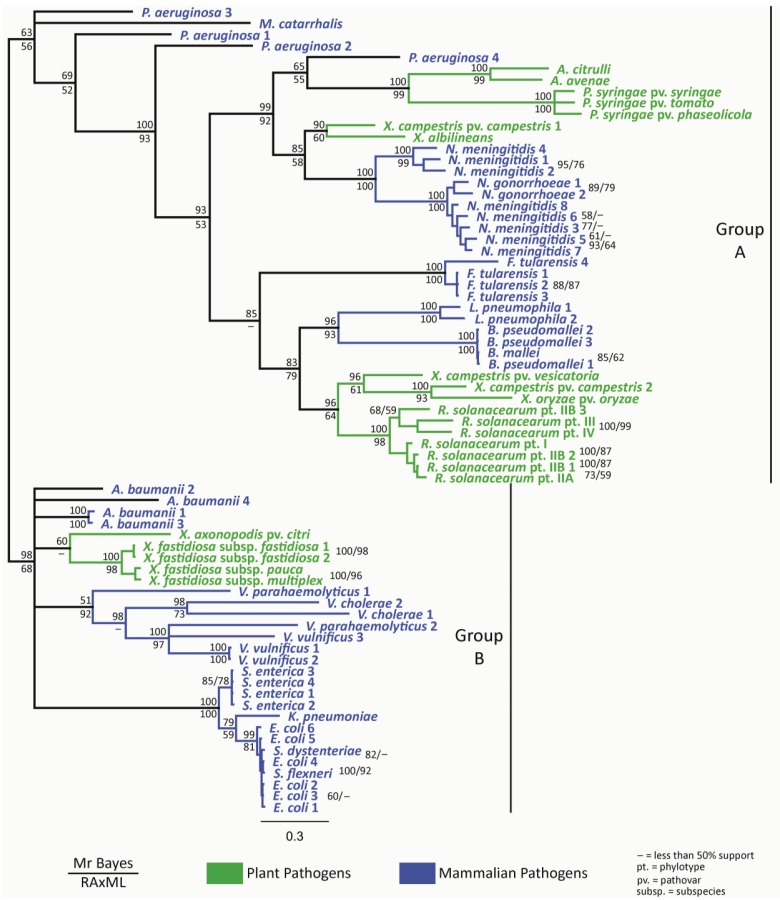
Our phylogenetic analyses distinguish between the two pilin groups previously defined for mammalian pathogens, namely group A and group B (type IVa and type IVb pilins; see above). Mammalian pathogens identified as belonging to group A (P. aeruginosa, Neisseria spp. and Moraxella spp.) and group B (E. coli, S. enterica and V. cholerae) [5,60] are separated into two major clades in our phylogeny (Figure 2). Pilin gene sequences from plant pathogenic bacteria were found to vary widely, but they do not form a group that is phylogenetically distinct from mammalian pathogens. Moreover, they can be clearly categorized as belonging to one of the two separate clades (Figure 2). In other words, the phylogenetic clades supported in our study can be used to infer the T4P group identity of the analyzed plant pathogens. The majority of plant pathogenic bacteria cluster with group A, which is the most diverse of the two pilin groups.
Figure 2.
Phylogeny of the unique pilA (pilin) DNA sequences (n = 71) found among the complete genomes of plant and mammalian bacterial pathogens deposited in GenBank (Table S1, supplementary material) as inferred by Bayesian phylogenetic (BP) analyses. Nodes with <50% support were collapsed. BP posterior probabilities and maximum likelihood (ML) bootstrap values are presented above and below each node, respectively. Dashes indicate relationships not supported based on ML analyses. Group A contains bacteria with type IVa pili and Group B contains bacteria with type IVb pili. Plant pathogens are in green, and mammalian pathogens are in blue.
A trend was observed where plant pathogen isolates from the same genus or species belong to the same distinct clade, which is also true for mammalian pathogens. So, as might be expected, the pilin gene from one isolate is generally more similar to that of an isolate from the same genus or species than from another genus or species. There are two main exceptions to this trend. One exception is the Xanthomonas genus, where the four Xanthomonas species represented here do not group together in any clear way, showing that the pilA sequence does not clearly differentiate members of this genus. Specifically for Xanthomonas campestris pv. campestris, the two available pilA alleles from different isolates of the same pathovar belong to different clades. Xanthomonads are by far the most diverse group in terms of pilA sequence examined here, and this probably reflects the wide range of plant hosts infected by different Xanthomonas species and pathovars and the high genetic diversity that occurs among members of this genus, particularly within X. campestris pv. campestris [91]. However, most intriguing was the finding that the pilin gene sequence from the Xanthomonas axonopodis isolate used in this study clustered in pilin group B, in contrast to the other xanthomonad pilin genes that clustered within group A.
The other exception to this trend is the genus Pseudomonas. Pilin genes from the plant pathogen Pseudomonas syringae and the mammalian pathogen Pseudomonas aeruginosa do not cluster together by genus in phylogenetic analyses, but isolates within each species do cluster together. This indicates the role that host specificity can play in pilin sequence variability. Because T4P are highly involved in virulence, it may be that pilA is important for host adaptation, causing this gene to be fairly divergent in members of the same genus because of the diversity among host organisms.
Another interesting finding from these phylogenetic analyses was that the X. axonopodis isolate and Xylella fastidiosa isolates were the only plant pathogenic bacteria belonging to the group B pilin clade. X. axonopodis and X. fastidiosa are predominantly pathogens of woody plants, while the other plant pathogens assessed here (which all possess a group A pilin sequence) tend to infect herbaceous plants. Further experimentation is necessary to understand the biological function and significance of the different types of plant pathogenic bacterial pilin sequences delineated in these analyses and determine the effects of host specificity on T4P.
4. The Role of T4P in Pathogenicity of Plant Pathogenic Bacteria
In contrast to the well-established role played by T4P in pathogenicity of several animal pathogenic bacteria, the role of T4P in pathogenicity of plant pathogenic bacteria is poorly understood. In plant pathogens, the contributions of T4P to virulence have been investigated mainly in vascular pathogens, particularly in those possessing the ability to colonize and spread via the plant xylem vessels. It has been proposed that T4P may contribute to bacterial colonization and spread in the xylem through cell attachment, biofilm formation and twitching motility. Nevertheless, other reports have also demonstrated the involvement of T4P in epiphytic fitness and, recently, in virulence of non-vascular plant pathogenic bacteria. In this section we summarize the current knowledge of the role of T4P in the interactions between plant pathogenic bacteria and their host plants as well as in important fitness properties.
4.1. Ralstonia solanacearum
Although twitching motility was described in the mid-1970s [92], and despite a few reports on T4P of plant pathogenic bacteria during the 1980s and 1990s, the first study to demonstrate a substantial role of twitching motility in virulence of a vascular plant pathogenic bacterium was only published in 2001 [93]. This study was of the soil-borne bacterium Ralstonia solanacearum, which is widely distributed in tropical, subtropical and warm temperate regions. It causes bacterial wilt disease in a wide range of plants, infecting more than 200 plant species that belong to more than 50 botanical families [94]. R. solanacearum invades plant roots, colonizes the xylem vessels and spreads rapidly to the upper parts of the plant via the vascular system. Typical symptoms of the disease include browning of the xylem, foliar yellowing and lethal wilting. In the aforementioned pioneer study on plant pathogenic bacterium T4P, Liu and colleagues observed twitching motility in agar plates by microscopically viewing the fringe surrounding growing colonies. Twitching motility was abolished in pilQ and pilT R. solanacearum mutants, which also induced slower and less severe wilting symptoms in tomato relative to the wild type [93].
The same research group further studied the role of T4P by creating pilA mutants of R. solanacearum [95]. These mutants were affected in many pathogenicity-related phenotypes including reduced virulence in tomato plants, reduced autoaggregation and biofilm formation in culture, and lack of attachment ability to tobacco cells in culture suspensions and to tomato roots. The pilA mutants were also not competent for transformation, illustrating for the first time the multiple functions of T4P in a phytopathogen.
In their first report, Liu and colleagues noted that twitching motility in R. solanacearum was only observed during the first day of growth on agar plates and was no longer detected after two days [93]. Later, Kang and colleagues [95] showed that pilA gene expression was reduced at high cell densities in the wild-type strain, while mutants in the transcriptional regulator PhcA were able to keep high levels of pilA expression and twitching motility at high cell densities. The response regulator PehR, which is repressed by PchA, positively influences pilA expression. Cell density-dependent regulation of twitching motility may be important for the life cycle of R. solanacearum, which lives in the soil and inside the vascular system of the plant host, where it causes disease by forming biofilm and obstructing water flow inside the plant. While during the soil-inhabiting phase swimming motility by means of flagella may be crucial to respond to and move towards gradients of seed/root exudates and to start the infection process, once inside the confined environment of the xylem vessels, twitching ability may be important to allow the bacterium to spread and colonize other parts of the infected plant and overcome nutrient limitations [95].
4.2. Xylella fastidiosa
Among plant pathogenic bacteria, Xylella fastidiosa has been the most investigated in terms of T4P functions. X. fastidiosa is a xylem-limited, non-flagellated bacterium that causes diseases in many crops including grape, citrus, peach, plum, almond [96] and blueberry [97]. This bacterium, found mainly in the warmer regions of the Americas, causes important diseases such as Pierce's Disease (PD) of grapevines, which is prevalent in the US, and citrus variegated chlorosis (CVC) in citrus, occurring mainly in Brazil. X. fastidiosa forms biofilms inside plant xylem vessels, and this is believed to be responsible for obstruction of water and nutrient flow to the rest of the plant, causing disease symptoms such as leaf scorch and chlorosis, among others. The bacterium is transmitted among plants by sharpshooter insects that feed on the xylem fluid and carry the bacterium in their mouth parts. Once injected into the xylem, the bacterium moves acropetally with the xylem fluid flow, and basipetally against the flow via twitching motility [98].
That X. fastidiosa produces T4P was evidenced by in silico analysis of the full genome, where 25 putative pil genes were shown to be present [99], and proteomic analysis that detected PilY1, PilT, and other T4P components [100]. Confirmation that X. fastidiosa can move against the direction of fluid flow was elegantly proven by experiments using microfluidic flow chambers (MFCs) that mimic the plant xylem vessels [98]. Upstream twitching motility explained the development of symptoms basipetally from the point of inoculation. Using MFCs, it was shown that the substrate adhesion force exerted by the wild-type strain (∼147 pN) far exceed the shear force of media flow (∼80 fN) [98,101], assuring that cells are powerful enough to move against the xylem fluid flow.
In X. fastidiosa, 1.0 to 5.8 μm long T4P are positioned at one of the cell's poles, while in the same pole, shorter (0.4 to 1.0 μm long) type I pili (T1P) are also present [98,102] (Figure 3). Mutational analysis of a T4P gene cluster showed that mutants in pilB and pilT [98], as well as in fimT, pilX and pilO [102], did not form T4P and were non-motile. Some of these mutants were not able to colonize upstream regions of grapevines [98]. Infection studies of grape plants in the greenhouse showed that T4P defective mutants were still infective, although symptoms were delayed and localized at the point of inoculation and did not spread along the vines [103]. Mutants in pilY1, believed to produce an adhesin located at the tip of T4P, showed reduced motility [102,104]. MFC studies showed that the speed of twitching motility against the flow of pilY1 defective mutants was one third of that of the wild type [104]. These data suggest that lack of PilY1 may cause cells to “slip”, compromising efficient attachment to surfaces and slowing the rates of twitching motility. In the same study, a mutant in T1P moved seven times faster than the wild type. T1P is important for efficient attachment to surfaces, and seems to restrict the speed of T4P-mediated twitching motility in X. fastidiosa [104].
Figure 3.
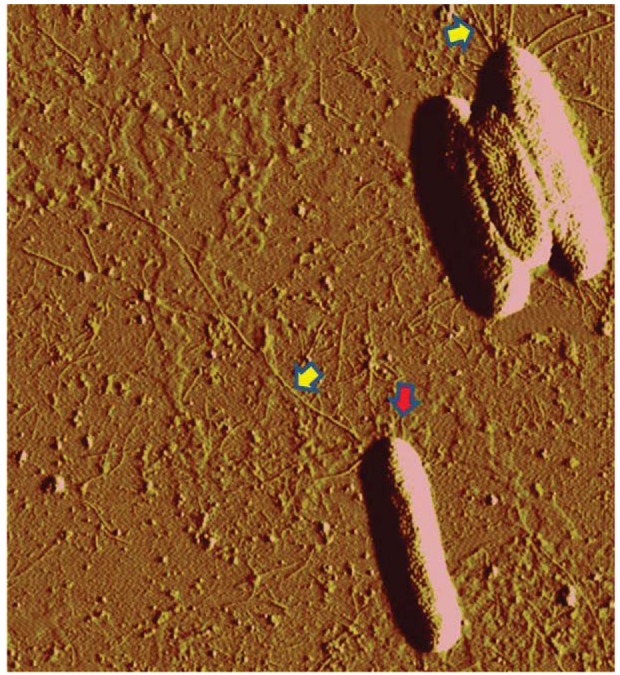
Atomic force micrograph of Xylella fastidiosa “Temecula” wild-type cells. Yellow arrows indicate T4P, while red arrows indicate shorter type I pili. Both types of pili are present at the same cell pole.
Several X. fastidiosa T4P mutants showed increased biofilm production [102], contrary to the concept that T4P contribute to the biofilm formation process. These results could be explained by a higher density of cells starting biofilms, achieved by a stronger attachment to surfaces by T1P in the absence of T4P. In other words, longer T4P likely disturb T1P contact with surfaces; therefore, in T4P deficient mutants, T1P can promote a stronger attachment to the surface and a consequent enhanced biofilm formation relative to the wild type [105].
Several proteins have been shown to have a regulatory role in function of T4P in X. fastidiosa. One example is TonB1, a protein located at the cytoplasmic membrane that is known to be involved in generating proton motive force for transport of iron-siderophore complexes and vitamin B12 across the outer membrane. A tonB1 mutant lost twitching motility although T4P was still produced [106]. This mutant also showed reduced biofilm formation in vitro and caused reduced PD symptoms in grapevines [106]. It was hypothesized that TonB1 may be involved in providing energy for import of molecules into the cell that may be involved in twitching motility, other than the molecules involved in T4P assembly. PilS/PilR is a two-component regulatory system that senses environmental signals by the sensor protein PilS, which later phosphorylates PilR that in turn regulates several processes, including production of T4P. pilR mutants of X. fastidiosa are non-motile and do not form T4P [102]. X. fastidiosa was also shown to have a pil-chp operon homolog to chemosensory systems in other bacteria [107]. One of the genes in this operon is pilL, which is homologous to the transmembrane chemoreceptor cheA in E. coli. A X. fastidiosa pilL mutant was shown to lose twitching motility, though it still formed T4P. This mutant also possessed decreased biofilm formation ability and induced delayed and less severe symptoms in grapevines relative to the wild type. The authors conclude that the chemosensory system in X. fastidiosa contributes to virulence, probably by regulating T4P function [107].
Different chemical compounds have been shown to affect twitching motility in X. fastidiosa. Removal or reduction of BSA from solid media was shown to increase movement in more than a dozen X. fastidiosa strains isolated from grape in different geographic areas [108]. The cell-to-cell signaling system mediated by the diffusible signal factor (DSF) is known to regulate several virulence traits in X. fastidiosa [109], including biofilm formation and production of hydrolytic enzymes. A mutant defective in production of DSF showed faster twitching motility than the wild type, while addition of purified DSF to the media greatly reduced twitching motility in the wild type [110]. DSF was shown to positively regulate the T1P gene fimA [111]; therefore, the increase in surface attachment by T1P may explain the observed negative effect on twitching motility by DSF, since a connection between T1P and reduction of twitching motility was previously established [104]. These results support the hypothesis that DSF modulates twitching motility and are in agreement with the main function proposed for DSF, which is to impact the transition between plant and insect host lifestyles [112], when cells have to choose between exploring new environments or attaching strongly to surfaces. Regarding metallic ions, iron is an environmental signal for modulating the transcriptional control of T4P genes during X. fastidiosa colonization: growth under low iron conditions was shown to increase the expression of several pil genes [113]. Increases in calcium concentration were shown to enhance twitching motility in agar plates and movement speed inside MFCs [114].
Several studies used gene expression analysis to dissect factors involved in control of pil gene expression. Growth of X. fastidiosa in grape xylem fluid led to an upregulation of genes involved in twitching motility such as fimT, pill, pilU and pilY1 [115]. Other studies showed that the PD1926 open reading frame, situated in the pil cluster and suggested to be involved in T4P formation, was under negative control by the alternate sigma factor AlgU [116], while it was positively controlled by GacA [117]. The role of PD1926 on twitching motility has not yet been proven. The lack of the global σ54 regulator RpoN was shown to downregulate the expression of one out of the five pilA paralogues in a non-pathogenic strain of X. fastidiosa [118].
No role for T4P has been assigned in the interactions of X. fastidiosa with insect vectors. pilB and fimA/pilO mutants were not affected in attachment to polysaccharides present in the vector mouth nor insect foregut extracts [119]. Moreover, when cells were grown in a media that facilitates acquisition by insects, pilY1 was downregulated, while genes involved in other attachment structures such as T1P were upregulated [120]. These observations suggest that T4P are not important for the interaction between X. fastidiosa and insect vectors. Accordingly, ongoing research is showing that T4P mutants are not impaired in bacterial transmission by insects to plant host [121].
4.3. Acidovorax citrulli
Acidovorax citrulli (formerly Acidovorax avenae subsp. citrulli) is a Gram-negative bacterium that infects different plants of the Cucurbitaceae family, mainly watermelon and melon [122]. Young seedlings and fruits are extremely susceptible to this pathogen, with fruit blotch and seedling blight, respectively, being the typical symptoms caused by this bacterium. A. citrulli is able to colonize and spread through vascular tissues of host plants [37,123], and it seems that this ability is important for induction of seedling blight and death, at least in melon plants [37].
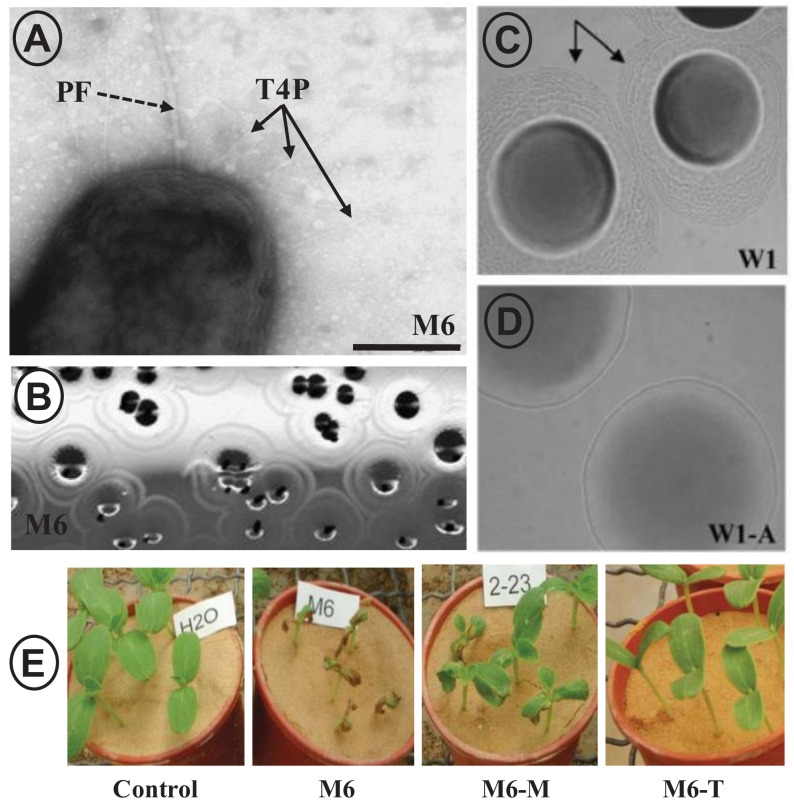
Individual mutations in pilM, pilA and pilT genes revealed that A. citrulli depends on the T4P machinery for twitching motility on semisolid media (shown for pilA in Figure 4). T4P were also shown to be required for the formation of wild-type levels of biofilm on plastic and glass surfaces [37]. MFC studies with TFP deficient mutants of A. citrulli further emphasized the importance of T4P for xylem colonization. Under the pressure of shear force from medium flowing through the microscopic channels, T4P were shown to be crucial for surface attachment. T4P mutants were unable to form biofilm in this environment, while wild-type cells formed dense biofilms that covered the whole surface of the chambers within 48–96 h (supplementary movie 2) [124]. Similar to what was shown for X. fastidiosa, T4P also facilitate downstream migration of A. citrulli via twitching motility [37,124]. Consistent with these in vitro assays showing the importance of T4P for various abilities of A. citrulli, T4P mutants were severely impaired in virulence on melon seedlings (Figure 4) following seed or stem inoculation. In both assays, significant differences in the percentage of dead seedling were observed between T4P mutants and the wild type. Moreover, in planta downward migration assays also supported the results from MFC experiments showing that T4P are important for efficient migration in the plant vascular system [37]. In contrast, no significant differences in symptom induction ability and growth in planta have been observed between a pilM mutant that does not produce T4P and the wild-type strain, following foliage inoculation of melon plants by both dip and spray methods [125]. These findings suggest that, in contrast to their importance of T4P for vascular infection, T4P and twitching motility may not have a crucial role in local, foliar infection by A. citrulli. Whether T4P significantly contribute to fruit infection by this pathogen is a subject that has not yet been investigated.
Figure 4.
T4P in Acidovorax citrulli, causal agent of bacterial fruit blotch and seedling blight of cucurbit plants. (A) Transmission electron microscopy of wild-type strain M6 following growth for 48 h on nutrient agar (NA) plates. Solid and dashed arrows indicate T4P and polar flagellum (PF), respectively (bar = 0.5 μm); (B) Typical twitching halos surrounding bulk colonies of strain M6 after 96 h of growth on NA; seen by the naked eye; (C) Twitching halos seen around colonies of strain W1 by light microscopy after 96 h of growth on NA; (D) Halos are not detected around colonies of a W1 pilA mutant (strain W1-A; lacking T4P and twitching motility) grown under similar conditions as described for W1 in C; (E) Effects of T4P deficiency on virulence of A. citrulli, assessed by seed-transmission assays with melon cv. Ophir. Seeds were incubated with the different strains (at 106 cfu/mL), and sowed in pots containing sand that were kept in a greenhouse at 25–28 °C. Pictures were taken 8 days after sowing. M6, wild type; M6-M, pilM mutant; M6-T, pilT mutant; control, seedlings from non-inoculated seeds. Figure composed from figures published by Bahar and colleagues [37].
Recent characterization of polar flagellum mutants of A. citrulli revealed that polar flagella contribute to the virulence of this bacterium following seed, stem and foliage inoculation [126]. Interestingly, polar flagellum mutants were not affected in biofilm formation ability, but they did show significantly reduced twitching motility in agar plates relative to the wild type. Transmission electron microscopy revealed that the polar flagellum mutants were not affected in their ability to produce T4P [126]. On the other hand, T4P mutants were previously shown to possess significantly altered swimming ability in comparison with the wild type: while the pilM mutant, lacking T4P, swims faster than the wild type, the hyperpiliated pilT mutant is significantly impaired in swimming motility [37]. These findings and the fact that T4P and polar flagella co-locate at one of the cell poles of the bacterium suggest that T4P might mechanically disturb swimming via the polar flagellum. Nevertheless, other types of interactions between these two organelles, including at regulatory levels, cannot be discarded.
4.4. Xanthomonas Species
The Xanthomonas genus contains more than 100 different plant pathogens that cause disease in over 300 different plant species. Surprisingly, the contribution of T4P to virulence and fitness has been studied in few xanthomonads. Some xanthomonads are vascular pathogens, while others infect the plant tissue locally. T4P mutants of the vascular pathogens X. oryzae pv. oryzae (Xoo; bacterial leaf blight of rice) and X. campestris pv. campestris (Xcc; black rot disease of cruciferous plants) were shown to possess reduced virulence relative to their parental strains. pilQ mutants of Xoo lack twitching motility, are deficient in biofilm formation and possess reduced virulence [127-129], while a pilA mutant of Xcc was shown to be negatively affected in surface motility and, to some extent, in virulence [130].
In contrast to Xoo and Xcc, X. campestris pv. vesicatoria (Xcv; bacterial spot disease of tomato and pepper) does not possess vascular colonization ability. Ojanen-Reuhs and colleagues [131] characterized a fimA (pilin) mutant of Xcv and showed that, despite clear impairments of this mutant in aggregate formation, adherence to leaf trichomes and resistance to UV radiation, no differences were observed between this mutant and the wild-type strain in symptom induction and growth in host plants following both infiltration and foliage spray inoculations. Van Doorn and colleagues [132] showed that X. hyacinthi cells (yellow disease of hyacinth) and purified type T4P attach to stomata of hyacinth leaves and suggested a role for T4P in the initial stages of infection of hyacinth leaves by this pathogen; however, this hypothesis was not further examined.
Significant contribution of T4P to virulence was recently demonstrated for the non-vascular pathogen X. oryzae pv. oryzicola (Xoc; bacterial leaf streak of rice) following a virulence screen of a collection of transposon mutants. Transposon insertions that yielded Xoc mutants with reduced virulence were identified in pilY, pilQ, pilM, pilZ and pilT genes. While for most of these mutants the reduction in virulence was slight to moderate, the pilT mutant was severely affected in this feature. [133]. To the best of our knowledge, Xoc is the only nonvascular xanthomonad for which a significant contribution of T4P to virulence has been demonstrated to date.
4.5. Pseudomonas Species
The Pseudomonas genus contains several plant pathogenic species of which P. syringae, comprising more than 50 different pathovars, is the most economically important [134]. As with xanthomonads, the involvement of P. syringae T4P in plant-pathogen interactions has been investigated in very few pathovars, with most of the reports being published over a decade ago.
P. syringae pv. phaseolicola (Psp; halo blight of common bean) was the first P. syringae pathovar to be investigated in terms of the role of T4P in plant-pathogen interactions. Romantschuk and colleagues [135] compared the leaf attachment ability of different Psp strains with natural variation in piliation. Leaf attachment assays showed a positive correlation between the degree of piliation and leaf adherence when bacteria were spray-inoculated onto the leaf surface. In addition, piliated bacteria appeared to attach more specifically to leaf stomata, while non-piliated bacteria attached more uniformly to the leaf surface, suggesting a role of T4P in attachment to stomata, as also inferred by the aforementioned study of Van Doorn and colleagues [132] with X. hyacinthi. When the bacterial inoculum was introduced by either stabbing or infiltration into bean leaves, no differences in symptom severity were observed among the different strains, suggesting a negligible contribution of T4P to virulence of this pathogen at post-penetration stages. However, clear differences among strains with different levels of piliation were observed when bacteria were spray-inoculated onto the leaf surface. In these assays, piliation and symptom induction ability correlated positively, while non-piliated strains were unable to induce visible symptoms, suggesting an important role of T4P for invasion into the leaf tissue [135].
Piliation was also shown to be important for initial adhesion and colonization of leaves by another common bean pathogen, Pseudomonas syringae pv. syringae (Psy; brown spot disease) [136]. However, no pathogenicity assays to assess the contribution of T4P to virulence of Psy were reported. Characterization of a pilA non-piliated mutant of P. syringae pv. tomato (Pto; bacterial speck disease of tomato) revealed that T4P contribute to UV tolerance in laboratory conditions and epiphytic fitness in planta. However, no differences were observed between the pilA mutant and the wild-type strain in their ability to induce disease symptoms on susceptible tomatoes following direct infiltration or foliage spray inoculation. In contrast to the aforementioned common bean pathovars, only small and apparently non-consistent differences were found between the pilA mutant and the Pto wild type in leaf attachment assays [137].
Recently, the role of T4P in plant-pathogen interactions was also examined in Pseudomonas syringae pv. tabaci (Pta; wildfire disease of tobacco) by Taguchi and Ichinose [138]. Surprisingly, the authors reported that Pta mutants impaired in pilA and pilO retained T4P-mediated twitching motility, although swimming and swarming motilities were attenuated in both mutants. The mutants were also shown to possess reduced biofilm formation ability in vitro relative to the wild type. In pathogenicity assays, no differences between the T4P mutants and the wild type were observed when inocula were directly infiltrated into leaves. However, the T4P mutants were shown to be significantly injured in terms of symptom induction and bacterial multiplication following foliage dip inoculation. Interestingly, the T4P mutants also had reduced expression of hrp genes and were slower than the wild type in hypersensitive response (HR) induction in non-host plants, suggesting co-regulation of T4P and the type III secretion machinery in this pathogen [138].
5. Conclusions
Investigations into the involvement of T4P in the interactions of plant pathogenic bacteria with their plant hosts are in the early stages, with most knowledge on this subject being accumulated only during the last decade. For some plant pathogenic bacterial species, it has been demonstrated that T4P play an important role in pathogenicity. T4P are important virulence factors in most evaluated vascular plant pathogenic bacteria. Findings from different vascular pathogen-host systems, summarized here, indicate that twitching motility and biofilm formation promoted by T4P are crucial for spread and colonization inside host xylem vessels. Recently, a significant contribution of T4P to virulence has been reported for a few non-vascular bacteria (like X. oryzae pv. oryzicola and P. syringae pv. tabaci). In some other non-vascular plant pathogenic bacteria, although T4P were not found to contribute to virulence, they were shown to promote epiphytic ability and survival under different stresses. Though not directly related to virulence, it is logical to assume that these features are important for bacterial infection under field conditions. Moreover, the possibility that T4P directly contribute to virulence in these pathogens cannot be completely discarded, as this issue has not been thoroughly investigated, and it is known that many virulence factors in plant pathogenic bacteria possess a subtle phenotype that may not be detected in artificial inoculation conditions [139].
Genetic and genomic studies of plant pathogenic bacteria have revealed that T4P genes are conserved and homologous with those of animal pathogenic bacteria. As shown in this study, phylogenetic analyses of pilin sequences do not discriminate between plant and animal pathogenic bacteria. T4P of plant- and animal-associated bacteria are also functionally conserved. For most plant pathogenic bacteria, however, there are many open questions regarding the importance of the functions and processes conferred by T4P to virulence and fitness. Other relevant questions deserving further attention include: Under which conditions and at which stages of infection are T4P genes expressed? Which genetic and environmental factors regulate the expression of T4P genes and T4P function? How does T4P expression and function interact with other virulence determinants like polar flagella, extracellular polysaccharides, type III secretion, multidrug resistance and others to promote plant infection and disease? Elucidation of these questions will contribute to a better understanding of the role played by T4P in the interactions between plant pathogenic bacteria and plants. Due to the importance of these organelles for colonization, survival and virulence (depending on the study organism), advances in this area may lead to the development of new strategies to combat these pathogens for the benefit of agriculture.
Acknowledgments
The investigation on virulence determinants of Acidovorax citrulli in the laboratory of S.B. has been supported by grants 823018005 from the Chief Scientist of the Israeli Ministry of Agriculture, and 4216-09 from the US-Israel Binational Agricultural Research and Development Fund (BARD). The phylogenetic analysis work at the L.D.L.F. laboratory was supported by the College of Agriculture and the Department of Entomology and Plant Pathology at Auburn University. We would like to acknowledge Justin Havird for advices on phylogenetic analyses and Harvey Hoch, Tom Burr, Steve Lindow, Rodrigo Almeida, Subhadeep Chatterjee, Nabil Killliny, Luisa Cruz, Paul Cobine and Ram Kumar Shrestha for sharing unpublished data for this review.
Supplementary Material
Table S1.
Bacterial pathogen complete genomes from GenBank used to obtain pilA DNA sequences used in pilA phylogeny (Figure 2).
| Phylogeny Name a | Genus | Species | Rank under species b | Isolate | Accession Number |
|---|---|---|---|---|---|
| A. avenae | Acidovorax | avenae | - | ATCC 19860 | CP002521 |
| A. citrulli | Acidovorax | citrulli | - | AAC00-1 | CP000512 |
| A. baumanii 1 | Acinetobacter | baumanii | - | ACICU | CP000863 |
| A. baumanii 2 | Acinetobacter | baumanii | - | ATCC 17978 | CP000521 |
| A. baumanii 3 | Acinetobacter | baumanii | - | TCDC-AB0715 | CP002522 |
| A. baumanii 4 | Acinetobacter | baumanii | - | AB0057 | CP001182 |
| B. mallei | Burkholderia | mallei | - | NCTC 10247 | CP000548 |
| B. pseudomallei 1 | Burkholderia | pseudomallei | - | 1106a | CP000572 |
| B. pseudomallei 2 | Burkholderia | pseudomallei | - | MSHR346 | CP001408 |
| B. pseudomallei 3 | Burkholderia | pseudomallei | - | 668 | CP000570 |
| E. coli 1 | Escherichia | coli | - | 55989 | CU928145 |
| E. coli 2 | Escherichia | coli | sv. O111:H | 11128 | AP010960 |
| E. coli 3 | Escherichia | coli | - | UMNK88 | CP002729 |
| E. coli 4 | Escherichia | coli | sv. O157:H7 | TW14359 | CP001368 |
| E. coli 5 | Escherichia | coli | - | UM146 | CP002167 |
| E. coli 6 | Escherichia | coli | sv. O83:H1 | NRG 857C | CP001855 |
| F. tularensis 1 | Francisella | tularensis | holarctica | FTNF002-00 | CP000803 |
| F. tularensis 2 | Francisella | tularensis | holarctica | OSU18 | CP000437 |
| F. tularensis 3 | Francisella | tularensis | mediasiatica | FSC147 | CP000915 |
| F. tularensis 4 | Francisella | tularensis | novicida | U112 | CP000439 |
| K. pneumoniae | Klebsiella | pneumoniae | - | 342 | CP000964 |
| L. pneumophila 1 | Legionella | pneumophila | - | 2300/99 Alcoy | CP001828 |
| L. pneumophila 2 | Legionella | pneumophila | - | Corby | CP000675 |
| M. catarrhalis | Moraxella | catarrhalis | - | RH4 | CP002005 |
| N. gonorrhoeae 1 | Neisseria | gonorrhoeae | - | NCCP11945 | CP001050 |
| N. gonorrhoeae 2 | Neisseria | gonorrhoeae | - | TCDC-NG08107 | CP002440 |
| N. meningitidis 1 | Neisseria | meningitidis | sg. C | FAM18 | AM421808 |
| N. meningitidis 2 | Neisseria | meningitidis | - | G2136 | CP002419 |
| N. meningitidis 3 | Neisseria | meningitidis | - | M01-240355 | CP002422 |
| N. meningitidis 4 | Neisseria | meningitidis | - | WUE2594 | FR774048 |
| N. meningitidis 5 | Neisseria | meningitidis | - | MC58 | AE002098 |
| N. meningitidis 6 | Neisseria | meningitidis | - | 8013 | FM999788 |
| N. meningitidis 7 | Neisseria | meningitidis | sg. A | Z2491 | AL157959 |
| N. meningitidis 8 | Neisseria | meningitidis | - | M01-240149 | CP002421 |
| P. aeruginosa 1 | Pseudomonas | aeruginosa | - | LESB58 | FM209186 |
| P. aeruginosa 2 | Pseudomonas | aeruginosa | - | PA7 | CP000744 |
| P. aeruginosa 3 | Pseudomonas | aeruginosa | - | PAO1 | AE004091 |
| P. aeruginosa 4 | Pseudomonas | aeruginosa | - | UCBPPPA1 4 | AY049071 |
| P. syringae pv. syringae | Pseudomonas | syringae | pv. syringae | B728a | CP000075 |
| P. syringae pv. tomato | Pseudomonas | syringae | pv. tomato | DC3000 | AE016853 |
| P. syringae pv. phaseolicola | Pseudomonas | syringae | pv. phaseolicola | 1448A | CP000058 |
| R. solanacearum pt. IIB1 | Ralstonia | solanacearum | pt. IIB | IPO1609 | CU914168 |
| R. solanacearum pt. IIB2 | Ralstonia | solanacearum | pt. IIB | MolK2 | CU861906 |
| R. solanacearum pt. IIA | Ralstonia | solanacearum | pt. IIA | CFBP2957 | FP885897 |
| R. solanacearum pt. I | Ralstonia | solanacearum | pt. I | GMI1000 | AL646052 |
| R. solanacearum pt. IIB3 | Ralstonia | solanacearum | pt. IIB | Po82 | CP002819 |
| R. solanacearum pt. III | Ralstonia | solanacearum | pt. III | CMR15 | FP885895 |
| R. solanacearum pt. IV | Ralstonia | solanacearum | pt. IV | PSI07 | FP885906 |
| S. enterica 1 | Salmonella | enterica | enterica sv. Choleraesuis | SC-B67 | AE017220 |
| S. enterica 2 | Salmonella | enterica | enterica sv. Heidelberg | SL476 | CP001120 |
| S. enterica 3 | Salmonella | enterica | enterica sv. Newport | SL254 | CP001113 |
| S. enterica 4 | Salmonella | enterica | enterica sv. Typhimurium | UK-1 | CP002614 |
| S. dysenteriae | Shigella | dysenteriae | - | Sd197 | CP000034 |
| S. flexneri | Shigella | flexneri | - | 2002017 | CP001383 |
| V. cholerae 1 | Vibrio | cholerae | - | HE39 | AFOQ01000009 |
| V. cholerae 2 | Vibrio | cholerae | - | MJ-1236 | CO001485 |
| V. parahaemolyticus 1 | Vibrio | parahaemolyticus | - | RIMD 2210633 | BA000031 |
| V. parahaemolyticus 2 | Vibrio | parahaemolyticus | - | 10329 | AFBW01000001 |
| V. vulnificus 1 | Vibrio | vulnificus | - | CMCP6 | AE016795 |
| V. vulnificus 2 | Vibrio | vulnificus | - | MO6-24/O | CP002469 |
| V. vulnificus 3 | Vibrio | vulnificus | - | YJ016 | BA000037 |
| X. oryzae. pv. oryzae | Xanthomonas | oryzae | pv. oryzae | KACC10331 | AE013598 |
| X. albilineans | Xanthomonas | albilineans | - | GPE PC73 | FP565176 |
| X. axonopodis. pv. citri | Xanthomonas | axonopodis | pv. citri | 306 | AE008923 |
| X. campestris pv. campestris 1 | Xanthomonas | campestris | pv. campestris | 8004 | CP000050 |
| X. campestris pv. campestris 2 | Xanthomonas | campestris | pv. campestris | B100 | AM920689 |
| X. campestris pv. vesicatoria | Xanthomonas | campestris | pv. vesicatoria | 85-10 | AM039952 |
| X. fastidiosa subsp. fastidiosa 1 | Xylella | fastidiosa | fastidiosa | Temecula | AE009442 |
| X. fastidiosa subsp. fastidiosa 2 | Xylella | fastidiosa | fastidiosa | M23 | CP001011 |
| X. fastidiosa subsp. multiplex | Xylella | fastidiosa | multiplex | M12 | CP000941 |
| X. fastidiosa subsp. pauca | Xylella | fastidiosa | pauca | 9a5c | AE003849 |
Name in phylogenetic tree (Figure 2);
Ranks under species: subspecies, phylotype (pt.), pathovar (pv.), serovar (sv.) and/or serogroup (sg.).
Supplementary movie 1.
Twitching motility of Acidovorax citrulli strain M6 after 72 h of growth on a nutrient agar (NA; 1.5% agar) plate. The plate was placed under a Nikon Ti/U E20L80 microscope (Nikon Co.) and the fringe of a colony was monitored using a time-lapse movie with a DS-Qi1Mc digital camera and the NIS ELEMENENTS software (Nikon Co.) over ∼8 h. The leading edge of the colony, composed of a monolayer of cells, is moving outward, away from the center of the colony, via T4P-mediated twitching motility at an approximate rate of 0.6 μm min−1.
Supplementary movie 2.
Biofilm formation of Acidovorax citrulli strain W1 during growth inside microfluidic flow chambers. The time-lapse movie shows the formation of a dense biofilm, covering the whole surface of the chamber, during a period of ∼22 h. The movie starts 72 h after cells were introduced into the chamber. The beginning of the movie shows cells scattered throughout the surface of the chamber. The cells remain bound to the surface despite being under flow conditions (flow is from left to right at a rate of 0.25μL min−1) and propagate to form a densely packed biofilm over time.
ZIP-Document (ZIP, 15163 KB)
References
- 1.Nudleman E., Kaiser D. Pulling together with type IV pili. J. Mol. Microbiol. Biotechnol. 2004;7:52–62. doi: 10.1159/000077869. [DOI] [PubMed] [Google Scholar]
- 2.Pelicic V. Type IV pili: E pluribus unum? Mol. Microbiol. 2008;68:827–837. doi: 10.1111/j.1365-2958.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- 3.Jarrell K.F., McBride M.J. The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 2008;6:466–476. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- 4.Mattick J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 5.Craig L., Pique M.E., Tainer J.A. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2004;2:363–378. doi: 10.1038/nrmicro885. [DOI] [PubMed] [Google Scholar]
- 6.Lyczak J.B., Cannon C.L., Pier G.B. Establishment of Pseudomonas aeruginosa infection: Lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 7.Alm R.A., Mattick J.S. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene. 1997;192:89–98. doi: 10.1016/s0378-1119(96)00805-0. [DOI] [PubMed] [Google Scholar]
- 8.McBride M.J. Bacterial gliding motility: Multiple mechanisms for cell movement over surfaces. Annu. Rev. Microbiol. 2001;55:49–75. doi: 10.1146/annurev.micro.55.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Wall D., Kaiser D. Type IV pili and cell motility. Mol. Microbiol. 1999;32:1–10. doi: 10.1046/j.1365-2958.1999.01339.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser D. Coupling cell movement to multicellular development in myxobacteria. Nat. Rev. Microbiol. 2003;1:45–54. doi: 10.1038/nrmicro733. [DOI] [PubMed] [Google Scholar]
- 11.Fussenegger M., Rudel T., Barten R., Ryll R., Meyer T.F. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae—A review. Gene. 1997;192:125–134. doi: 10.1016/s0378-1119(97)00038-3. [DOI] [PubMed] [Google Scholar]
- 12.Craig L., Li J. Type IV pili: Paradoxes in form and function. Curr. Opin. Struct. Biol. 2008;18:267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen I., Dubnau D. DNA transport during transformation. Front. Biosci. 2003;8:S544–S556. doi: 10.2741/1047. [DOI] [PubMed] [Google Scholar]
- 14.Parge H.E., Forest K.T., Hickey M.J., Christensen D.A., Getzoff E.D., Tainer J.A. Structure of the fiber-forming protein pilin at 2.6-angstrom resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 15.Burrows L.L. Weapons of mass retraction. Mol. Microbiol. 2005;57:878–888. doi: 10.1111/j.1365-2958.2005.04703.x. [DOI] [PubMed] [Google Scholar]
- 16.Lautrop H. Bacterium anitratum transferred to the genus Cytophaga. Acta Pathol. Microbiol. Scand. Suppl. 1962;154:303–304. [PubMed] [Google Scholar]
- 17.Henrichsen J., Blom J. Correlation between twitching motility and possession of polar fimbriae in Acinetobacter calcoaceticus. Acta Pathol. Microbiol. Scand. B. 1975;83:103–115. doi: 10.1111/j.1699-0463.1975.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 18.Henrichsen J., Froholm L.O., Bovre K. Studies on bacterial surface translocation. 2. Correlation of twitching motility and fimbriation in colony variants of Moraxella nonliquefaciens, M. bovis, and M. kingii. Acta Pathol. Microbiol. Scand. B. 1972;80:445–452. [PubMed] [Google Scholar]
- 19.Henrichsen J. Occurrence of twitching motility among gram-negative bacteria. Acta Pathol. Microbiol. Scand. B. 1975;83:171–178. doi: 10.1111/j.1699-0463.1975.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 20.Henrichsen J. Bacterial surface translocation—Survey and a classification. Bacteriol. Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macrae T.H., McCurdy H.D. Evidence for motility-related fimbriae in gliding microorganism Myxococcus xanthus. Can. J. Microbiol. 1976;22:1589–1593. doi: 10.1139/m76-234. [DOI] [PubMed] [Google Scholar]
- 22.Bradley D.E. Function of Pseudomonas aeruginosa PAO polar pili—Twitching motility. Can. J. Microbiol. 1980;26:146–154. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- 23.Bradley D.E. Evidence for retraction of Pseudomonas aeruginosa RNA phage pili. Biochem. Biophys. Res. Commun. 1972;47:142–149. doi: 10.1016/s0006-291x(72)80021-4. [DOI] [PubMed] [Google Scholar]
- 24.Bradley D.E. Shortening of Pseudomonas aeruginosa pili after RNA-phage adsorption. J. Gen. Microbiol. 1972;72:303–319. doi: 10.1099/00221287-72-2-303. [DOI] [PubMed] [Google Scholar]
- 25.Merz A.J., So M., Sheetz M.P. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 26.Sun H., Zusman D.R., Shi W.Y. Type IV pilus of Myxococcus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 2000;10:1143–1146. doi: 10.1016/s0960-9822(00)00705-3. [DOI] [PubMed] [Google Scholar]
- 27.Skerker J.M., Berg H.C. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. USA. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Toole G., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 29.Farinha M.A., Conway B.D., Glasier L.M.G., Ellert N.W., Irvin R.T., Sherburne R., Paranchych W. Alteration of the pilin adhesin of Pseudomonas aeruginosa PAO results in normal pilus biogenesis but a loss of adherence to human pneumocyte cells and decreased virulence in mice. Infect. Immun. 1994;62:4118–4123. doi: 10.1128/iai.62.10.4118-4123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothbard J.B., Fernandez R., Wang L., Teng N.N.H., Schoolnik G.K. Antibodies to peptides corresponding to a conserved sequence of gonococcal pilins block bacterial adhesion. Proc. Natl. Acad. Sci. USA. 1985;82:915–919. doi: 10.1073/pnas.82.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruehl W.W., Marrs C., Beard M.K., Shokooki V., Hinojoza J.R., Banks S., Bieber D., Mattick J.S. Q-pili enhance the attachment of Moraxella bovis to bovine corneas in vitro. Mol. Microbiol. 1993;7:285–288. doi: 10.1111/j.1365-2958.1993.tb01119.x. [DOI] [PubMed] [Google Scholar]
- 32.Chi E., Mehl T., Nunn D., Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect. Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang P., Burrows L.L. Biofilm formation by hyperpiliated mutants of Pseudomonas aeruginosa. J. Bacteriol. 2003;185:2374–2378. doi: 10.1128/JB.185.7.2374-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 35.O'Toole G.A., Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 36.Heydorn A., Ersboll B., Kato J., Hentzer M., Parsek M.R., Tolker-Nielsen T., Givskov M., Molin S. Statistical analysis of Pseudomonas aeruginosa biofilm development: Impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl. Environ. Microbiol. 2002;68:2008–2017. doi: 10.1128/AEM.68.4.2008-2017.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahar O., Goffer T., Burdman S. Type IV pili are required for virulence, twitching motility, And biofilm formation of Acidovorax avenae subsp. citrulli. Mol. Plant Microbe Interact. 2009;22:909–920. doi: 10.1094/MPMI-22-8-0909. [DOI] [PubMed] [Google Scholar]
- 38.Wu S.S., Wu J., Kaiser D. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol. Microbiol. 1997;23:109–121. doi: 10.1046/j.1365-2958.1997.1791550.x. [DOI] [PubMed] [Google Scholar]
- 39.Klausen M., Heydorn A., Ragas P., Lambertsen L., Aaes-Jorgensen A., Molin S., Tolker-Nielsen T. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 2003;48:1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- 40.Conrad J.C., Gibiansky M.L., Jin F., Gordon V.D., Motto D.A., Mathewson M.A., Stopka W.G., Zelasko D.C., Shrout J.D., Wong G.C.L. Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys. J. 2011;100:1608–1616. doi: 10.1016/j.bpj.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collyn F., Lety M.A., Nair S., Escuyer V., Ben Younes A., Simonet M., Marceau M. Yersinia pseudotuberculosis harbors a type IV pilus gene cluster that contributes to pathogenicity. Infect. Immun. 2002;70:6196–6205. doi: 10.1128/IAI.70.11.6196-6205.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X.L., Tsui I.S.M., Yip C.M.C., Fung A.W.Y., Wong D.K.H., Dai X.Y., Yang Y.H., Hackett J., Morris C. Salmonella enterica serovar Typhi uses type IVB pili to enter human intestinal epithelial cells. Infect. Immun. 2000;68:3067–3073. doi: 10.1128/iai.68.6.3067-3073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comolli J.C., Hauser A.R., Waite L., Whitchurch C.B., Mattick J.S., Engel J.N. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 1999;67:3625–3630. doi: 10.1128/iai.67.7.3625-3630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stavrinides J., Ma W., Guttman D.S. Terminal reassortment drives the quantum evolution of type III effectors in bacterial pathogens. Plos Pathog. 2006;2:913–921. doi: 10.1371/journal.ppat.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang P.J., Hauser A.R., Apodaca G., Fleiszig S.M.J., Wiener-Kronish J., Mostov K., Engel J.N. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol. Microbiol. 1997;24:1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- 46.Yahr T.L., Wolfgang M.C. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 2006;62:631–640. doi: 10.1111/j.1365-2958.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- 47.Whitchurch C.B., Beatson S.A., Comolli J.C., Jakobsen T., Sargent J.L., Bertrand J.J., West J., Klausen M., Waite L.L., Kang P.J., et al. Pseudomonas aeruginosa fimL regulates multiple virulence functions by intersecting with Vfr-modulated pathways. Mol. Microbiol. 2005;55:1357–1378. doi: 10.1111/j.1365-2958.2005.04479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulcher N.B., Holliday P.M., Klem E., Cann M.J., Wolfgang M.C. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Mol. Microbiol. 2010;76:889–904. doi: 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sivaneson M., Mikkelsen H., Ventre I., Bordi C., Filloux A. Two-component regulatory systems in Pseudomonas aeruginosa: An intricate network mediating fimbrial and efflux pump gene expression. Mol. Microbiol. 2011;79:1353–1366. doi: 10.1111/j.1365-2958.2010.07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egerton J.R., Cox P.T., Anderson B.J., Kristo C., Norman M., Mattick J.S. Protection of sheep against footrot with a recombinant DNA-based fimbrial vaccine. Vet. Microbiol. 1987;14:393–409. doi: 10.1016/0378-1135(87)90030-7. [DOI] [PubMed] [Google Scholar]
- 51.Merz A.J., So M. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 2000;16:423–457. doi: 10.1146/annurev.cellbio.16.1.423. [DOI] [PubMed] [Google Scholar]
- 52.Hazlett L.D., Moon M.M., Singh A., Berk R.S., Rudner X.L. Analysis of adhesion, piliation, protease production and ocular infectivity of several Pseudomonas aeruginosa strains. Curr. Eye Res. 1991;10:351–362. doi: 10.3109/02713689108996341. [DOI] [PubMed] [Google Scholar]
- 53.Pujol C., Eugene E., Marceau M., Nassif X. The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion. Proc. Natl. Acad. Sci. USA. 1999;96:4017–4022. doi: 10.1073/pnas.96.7.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bieber D., Ramer S.W., Wu C.Y., Murray W.J., Tobe T., Fernandez R., Schoolnik G.K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 55.Averhoff B., Friedrich A. Type IV pili-related natural transformation systems: DNA transport in mesophilic and thermophilic bacteria. Arch. Microbiol. 2003;180:385–393. doi: 10.1007/s00203-003-0616-6. [DOI] [PubMed] [Google Scholar]
- 56.Lu H.M., Motley S.T., Lory S. Interactions of the components of the general secretion pathway: Role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol. Microbiol. 1997;25:247–259. doi: 10.1046/j.1365-2958.1997.4561818.x. [DOI] [PubMed] [Google Scholar]
- 57.Rudel T., Facius D., Barten R., Scheuerpflug I., Nonnenmacher E., Meyer T.F. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA. 1995;92:7986–7990. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jyot J., Ramphal R. Flagella and pili of Pseudomonas aeruginosa. In: Rehm B.H.A., editor. Pseudomonas: Model Organism, Pathogen, Cell Factory. Willey; Weinheim, Germany: 2008. pp. 85–108. [Google Scholar]
- 59.Hobbs M., Mattick J.S. Common components in the assembly of type-4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus—A general system for the formation of surface-associated protein complexes. Mol. Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 60.Strom M.S., Lory S. Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 61.Whitchurch C.B., Alm R.A., Mattick J.S. The alginate regulator AlgR and an associated sensor FimS are required for twitching motility in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1996;93:9839–9843. doi: 10.1073/pnas.93.18.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Toole G.A., Gibbs K.A., Hager P.W., Phibbs P.V., Kolter R. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2000;182:425–431. doi: 10.1128/jb.182.2.425-431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darzins A. The pilG gene-product, required for Pseudomonas aeruginosa pilus production and twitching motility, is homologous to the enteric, single-domain response regulator CheY. J. Bacteriol. 1993;175:5934–5944. doi: 10.1128/jb.175.18.5934-5944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darzins A. Characterization of a Pseudomonas aeruginosa gene-cluster involved in pilus biosynthesis and twitching motility—Sequence similarity to the chemotaxis proteins of enterics and the gliding bacterium Myxococcus xanthus. Mol. Microbiol. 1994;11:137–153. doi: 10.1111/j.1365-2958.1994.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 65.Darzins A. The Pseudomonas aeruginosa pilK gene encodes a chemotactic methyltransferase (CheR) homolog that is translationally regulated. Mol. Microbiol. 1995;15:703–717. doi: 10.1111/j.1365-2958.1995.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 66.Whitchurch C.B., Leech A.J., Young M.D., Kennedy D., Sargent J.L., Bertrand J.J., Semmler A.B.T., Mellick A.S., Martin P.R., Alm R.A., et al. Characterization of a complex chemosensory signal transduction which controls twitching motility in Pseudomonas aeruginosa. Mol. Microbiol. 2004;52:873–893. doi: 10.1111/j.1365-2958.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 67.Zusman D.R., Scott A.E., Yang Z., Kirby J.R. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat. Rev. Microbiol. 2007;5:862–872. doi: 10.1038/nrmicro1770. [DOI] [PubMed] [Google Scholar]
- 68.Patriquin G.M., Banin E., Gilmour C., Tuchman R., Greenberg E.P., Poole K. Influence of quorum sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J. Bacteriol. 2008;190:662–671. doi: 10.1128/JB.01473-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shrout J.D., Chopp D.L., Just C.L., Hentzer M., Givskov M., Parsek M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006;62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 70.Johnson T.L., Abendroth J., Hol W.G.J., Sandkvist M. Type II secretion: From structure to function. FEMS Microbiol. Lett. 2006;255:175–186. doi: 10.1111/j.1574-6968.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 71.Nunn D. Bacterial type II protein export and pilus biogenesis: More than just homologies? Trends Cell Biol. 1999;9:402–408. doi: 10.1016/s0962-8924(99)01634-7. [DOI] [PubMed] [Google Scholar]
- 72.Peabody C.R., Chung Y.J., Yen M.R., Vidal-Ingigliardi D., Pugsley A.P., Saier M.H. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiol. SGM. 2003;149:3051–3072. doi: 10.1099/mic.0.26364-0. [DOI] [PubMed] [Google Scholar]
- 73.Lory S., Strom M.S. Structure-function relationship of type-IV prepilin peptidase of Pseudomonas aeruginosa—A review. Gene. 1997;192:117–121. doi: 10.1016/s0378-1119(96)00830-x. [DOI] [PubMed] [Google Scholar]
- 74.Strom M.S., Nunn D.N., Lory S. A single bifunctional enzyme, Pild, catalyzes cleavage and N-methylation of proteins belonging to the type-IV pilin family. Proc. Natl. Acad. Sci. USA. 1993;90:2404–2408. doi: 10.1073/pnas.90.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bulyha I., Schmidt C., Lenz P., Jakovljevic V., Hone A., Maier B., Hoppert M., Sogaard-Andersen L. Regulation of the type IV pili molecular machine by dynamic localization of two motor proteins. Mol. Microbiol. 2009;74:691–706. doi: 10.1111/j.1365-2958.2009.06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitchurch C.B., Hobbs M., Livingston S.P., Krishnapillai V., Mattick J.S. Characterization of a Pseudomonas aeruginosa twitching motility gene and evidence for a specialized protein export system widespread in eubacteria. Gene. 1991;101:33–44. doi: 10.1016/0378-1119(91)90221-v. [DOI] [PubMed] [Google Scholar]
- 77.Bradley D.E. Adsorption of Pseudomonas aeruginosa pilus-dependent bacteriophages to a host mutant with nonretractile pili. Virology. 1974;58:149–163. doi: 10.1016/0042-6822(74)90150-0. [DOI] [PubMed] [Google Scholar]
- 78.Chiang P., Habash M., Burrows L.L. Disparate subcellular localization patterns of Pseudomonas aerufinosa type IV pilus ATPases involved in twitching motility. J. Bacteriol. 2005;187:829–839. doi: 10.1128/JB.187.3.829-839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Helm R.A., Barnhart M.M., Seifert H.S. pilQ missense mutations have diverse effects on PilQ multimer formation, piliation, and pilus function in Neisseria gonorrhoeae. J. Bacteriol. 2007;189:3198–3207. doi: 10.1128/JB.01833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kus J.V., Tullis E., Cvitkovitch D.G., Burrows L.L. Significant differences in type IV pilin allele distribution among Pseudomonas aeruginosa isolates from cystic fibrosis (CF) versus non-CIF patients. Microbiol. SGM. 2004;150:1315–1326. doi: 10.1099/mic.0.26822-0. [DOI] [PubMed] [Google Scholar]
- 81.Harvey H., Habash M., Aidoo F., Burrows L.L. Single-residue changes in the C-terminal disulfide-bonded loop of the Pseudomonas aeruginosa type IV pilin influence pilus assembly and twitching motility. J. Bacteriol. 2009;191:6513–6524. doi: 10.1128/JB.00943-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cehovin A., Winterbotham M., Lucidarme J., Borrow R., Tang C.M., Exley R.M., Pelicic V. Sequence conservation of pilus subunits in Neisseria meningitides. Vaccine. 2010;28:4817–4826. doi: 10.1016/j.vaccine.2010.04.065. [DOI] [PubMed] [Google Scholar]
- 83.Stewart R.M.K., Wiehlmann L., Ashelford K.E., Preston S.J., Frimmersdorf E., Campbell B.J., Neal T.J., Hall N., Tuft S., Kaye S.B., et al. Genetic characterization indicates that a specific sub-population of Pseudomonas aeruginosa is associated with keratitis infections. J. Clin. Microbiol. 2011;49:993–1003. doi: 10.1128/JCM.02036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luke-Marshall N.R., Sauberan S.L., Campagnari A.A. Comparative analyses of the Moraxella catarrhalis type-IV pilus structural subunit PilA. Gene. 2011;477:19–23. doi: 10.1016/j.gene.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 85.Whiley D.M., Jacobsson S., Tapsall J.W., Nissen M.D., Sloots T.P., Unemo M. Alterations of the pilQ gene in Neisseria gonorrhoeae are unlikely contributors to decreased susceptibility to ceftriaxone and cefixime in clinical gonococcal strains. J. Antimicrob. Chemother. 2010;65:2543–2547. doi: 10.1093/jac/dkq377. [DOI] [PubMed] [Google Scholar]
- 86.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 87.Drummond A.J., Ashton B., Cheung M., Cooper A., Duran C., Field M., Heled J., Kearse M., Markowitz S., Moir R., et al. 2011. Geneious v5.4. Available online: http://www.genious.com/ (accessed on 8 October 2011)
- 88.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 89.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 90.Stamatakis A., Hoover P., Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 91.Valverde A., Hubert T., Stolov A., Dagar A., Kopelowitz J., Burdman S. Assesment of genetic diversity of Xanthomonas campestris pv. campestris isolates from Israel by various DNA fingerprinting techniques. Plant Pathol. 2007;56:17–25. [Google Scholar]
- 92.Henrichsen J., Blom J. Examination of fimbriation of some gram-negative rods with and without twitching and gliding motility. Acta Pathol. Microbiol. Scand. B. 1975;83:161–170. doi: 10.1111/j.1699-0463.1975.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 93.Liu H.L., Kang Y.W., Genin S., Schell M.A., Denny T.P. Twitching motility of Ralstonia solanacearum requires a type IV pilus system. Microbiol. SGM. 2001;147:3215–3229. doi: 10.1099/00221287-147-12-3215. [DOI] [PubMed] [Google Scholar]
- 94.Genin S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 2010;187:920–928. doi: 10.1111/j.1469-8137.2010.03397.x. [DOI] [PubMed] [Google Scholar]
- 95.Kang Y.W., Liu H.L., Genin S., Schell M.A., Denny T.P. Ralstonia solanacearum requires type 4 pili to adhere to multiple surfaces and for natural transformation and virulence. Mol. Microbiol. 2002;46:427–437. doi: 10.1046/j.1365-2958.2002.03187.x. [DOI] [PubMed] [Google Scholar]
- 96.Hopkins D.L., Purcell A.H. Xylella fastidiosa: Cause of Pierce's disease of grapevine and other emergent diseases. Plant Dis. 2002;86:1056–1066. doi: 10.1094/PDIS.2002.86.10.1056. [DOI] [PubMed] [Google Scholar]
- 97.Chang C.J., Donaldson R., Brannen P., Krewer G., Boland R. Bacterial leaf corch, a new blueberry disease caused by Xylella fastidiosa. Hortscience. 2009;44:413–417. [Google Scholar]
- 98.Meng Y.Z., Li Y.X., Galvani C.D., Hao G.X., Turner J.N., Burr T.J., Hoch H.C. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 2005;187:5560–5567. doi: 10.1128/JB.187.16.5560-5567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Sluys M.A., de Oliveira M.C., Monteiro-Vitorello C.B., Miyaki C.Y., Furlan L.R., Camargo L.E.A., da Silva A.C.R., Moon D.H., Takita M.A., Lemos E.G.M., et al. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 2003;185:1018–1026. doi: 10.1128/JB.185.3.1018-1026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smolka M.B., Martins D., Winck F.V., Santoro C.E., Castellari R.R., Ferrari F., Brum I.J., Galembeck E., Coletta H.D., Machado M.A., et al. Proteome analysis of the plant pathogen Xylella fastidiosa reveals major cellular and extracellular proteins and a peculiar codon bias distribution. Proteomics. 2003;3:224–237. doi: 10.1002/pmic.200390031. [DOI] [PubMed] [Google Scholar]
- 101.De La Fuente L., Montanes E., Meng Y.Z., Li Y.X., Burr T.J., Hoch H.C., Wu M.M. Assessing adhesion forces of type I and type IV pili of Xylella fastidiosa bacteria by use of a microfluidic flow chamber. Appl. Environ. Microbiol. 2007;73:2690–2696. doi: 10.1128/AEM.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y.X., Hao G.X., Galvani C.D., Meng Y.Z., De la Fuente L., Hoch H.C., Burr T.J. Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell-cell aggregation. Microbiol. SGM. 2007;153:719–726. doi: 10.1099/mic.0.2006/002311-0. [DOI] [PubMed] [Google Scholar]
- 103.Hoch H., Burr T. Personal communication. Cornell University; Geneva, NY, USA: 2007. [Google Scholar]
- 104.De La Fuente L., Burr T.J., Hoch H.C. Mutations in type I and type IV pilus biosynthetic genes affect twitching motility rates in Xylella fastidiosa. J. Bacteriol. 2007;189:7507–7510. doi: 10.1128/JB.00934-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De La Fuente L., Burr T.J., Hoch H.C. Autoaggregation of Xylella fastidiosa cells is influenced by type I and type IV pili. Appl. Environ. Microbiol. 2008;74:5579–5582. doi: 10.1128/AEM.00995-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cursino L., Li Y.X., Zaini P.A., De La Fuente L., Hoch H.C., Burr T.J. Twitching motility and biofilm formation are associated with tonB1 in Xylella fastidiosa. FEMS Microbiol. Lett. 2009;299:193–199. doi: 10.1111/j.1574-6968.2009.01747.x. [DOI] [PubMed] [Google Scholar]
- 107.Cursino L., Galvani C.D., Athinuwat D., Zaini P.A., Li Y., De La Fuente L., Hoch H., Burr T.J., Mowery P. Identification of an operon, Pil-Chp, that controls twitching motility and virulence in Xylella fastidiosa. Mol. Plant Microbe Interact. 2011;24:1198–1206. doi: 10.1094/MPMI-10-10-0252. [DOI] [PubMed] [Google Scholar]
- 108.Galvani C.D., Li Y.X., Burr T.J., Hoch H.C. Twitching motility among pathogenic Xylella fastidiosa isolates and the influence of bovine serum albumin on twitching-dependent colony fringe morphology. FEMS Microbiol. Lett. 2007;268:202–208. doi: 10.1111/j.1574-6968.2006.00601.x. [DOI] [PubMed] [Google Scholar]
- 109.Chatterjee S., Newman K.L., Lindow S.E. Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant Microbe Interact. 2008;21:1309–1315. doi: 10.1094/MPMI-21-10-1309. [DOI] [PubMed] [Google Scholar]
- 110.Chatterjee, S.; Lindow, S.; De La Fuente, L.; Burr, T.; Hoch, H. Centre for DNA Fingerprinting and Diagnostics, Nampally, Hyderabad, India. Unpublished work, 2011
- 111.Chatterjee S., Wistrom C., Lindow S.E. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci. USA. 2008;105:2670–2675. doi: 10.1073/pnas.0712236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chatterjee S., Almeida R.P.P., Lindow S. Living in two worlds: The plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 2008;46:243–271. doi: 10.1146/annurev.phyto.45.062806.094342. [DOI] [PubMed] [Google Scholar]
- 113.Zaini P.A., Fogaca A.C., Lupo F.G.N., Nakaya H.I., Vencio R.Z.N., da Silva A.M. The iron stimulon of Xylella fastidiosa includes genes for type IV pilus and colicin V-like bacteriocins. J. Bacteriol. 2008;190:2368–2378. doi: 10.1128/JB.01495-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cruz, L.; Cobine, P.; De La Fuente, L. Auburn University, Auburn, AL, USA. Unpublished work, 2011
- 115.Shi X.Y., Bi J.L., Morse J.G., Toscano N.C., Cooksey D.A. Differential expression of genes of Xylella fastidiosa in xylem fluid of citrus and grapevine. FEMS Microbiol. Lett. 2010;304:82–88. doi: 10.1111/j.1574-6968.2009.01885.x. [DOI] [PubMed] [Google Scholar]
- 116.Shi X.Y., Dumenyo C.K., Hernandez-Martinez R., Azad H., Cooksey D.A. Characterization of regulatory pathways in Xylella fastidiosa: Genes and phenotypes controlled by algU. Appl. Environ. Microbiol. 2007;73:6748–6756. doi: 10.1128/AEM.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shi X.Y., Dumenyo C.K., Hernandez-Martinez R., Azad H., Cooksey D.A. Characterization of regulatory pathways in Xylella fastidiosa: Genes and phenotypes controlled by gacA. Appl. Environ. Microbiol. 2009;75:2275–2283. doi: 10.1128/AEM.01964-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neto J., Koide T., Abe C.M., Gomes S.L., Marques M.V. Role of σ54 in the regulation of genes involved in type I and type IV pili biogenesis in Xylella fastidiosa. Arch. Microbiol. 2008;189:249–261. doi: 10.1007/s00203-007-0314-x. [DOI] [PubMed] [Google Scholar]
- 119.Killiny N., Almeida R.P.P. Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Appl. Environ. Microbiol. 2009;75:521–528. doi: 10.1128/AEM.01921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Killiny N., Almeida R.P.P. Host structural carbohydrate induces vector transmission of a bacterial plant pathogen. Proc. Natl. Acad. Sci. USA. 2009;106:22416–22420. doi: 10.1073/pnas.0908562106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Killiny N., Almeida R. Personal communication. University of California; Berkeley, CA, USA: 2011. [Google Scholar]
- 122.Schaad N.W., Postnikova E., Sechler A., Claflin L.E., Vidaver A.K., Jones J.B., Agarkova I., Ignatov A., Dickstein E., Ramundo B.A. Reclassification of subspecies of Acidovorax avenae as A. avenae (Manns 1905) emend., A. cattleyae (Pavarino, 1911) comb. nov., A. citrulli Schaad et al., 1978) comb. nov., and proposal of A. oryzae sp. nov. Syst. Appl. Microbiol. 2008;31:434–446. doi: 10.1016/j.syapm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 123.Makizumi Y., Igarashi M., Gotoh K., Murao K., Yamamoto M., Udonsri N., Ochiai H., Thummabenjapone P., Kaku H. Genetic diversity and pathogenicity of cucurbit-associated. Acidovorax. J. Gen. Plant Pathol. 2011;77:24–32. [Google Scholar]
- 124.Bahar O., De La Fuente L., Burdman S. Assessing adhesion, biofilm formation and motility of Acidovorax citrulli using microfluidic flow chambers. FEMS Microbiol. Lett. 2010;312:33–39. doi: 10.1111/j.1574-6968.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 125.Bahar, O.; Shrestha, R.K.; Burdman, S. The Hebrew University of Jerusalem, Rehovot, Israel. Unpublished work, 2011
- 126.Bahar O., Levi N., Burdman S. The cucurbit pathogenic bacterium Acidovorax citrulli requires a polar flagellum for full virulence before and after host-tissue penetration. Mol. Plant Microbe Interact. 2011;24:1040–1050. doi: 10.1094/MPMI-02-11-0041. [DOI] [PubMed] [Google Scholar]
- 127.Das A., Rangaraj N., Sonti R.V. Multiple adhesin-like functions of Xanthomonas oryzae pv. oryzae are involved in promoting leaf attachment, entry, and virulence on rice. Mol. Plant Microbe Interact. 2009;22:73–85. doi: 10.1094/MPMI-22-1-0073. [DOI] [PubMed] [Google Scholar]
- 128.Lim S.H., So B.H., Wang J.C., Song E.S., Park Y.J., Lee B.M., Kang H.W. Functional analysis of pilQ gene in Xanthomanas oryzae pv. oryzae, bacterial blight pathogen of rice. J. Microbiol. 2008;46:214–220. doi: 10.1007/s12275-007-0173-9. [DOI] [PubMed] [Google Scholar]
- 129.Wang J.C., So B.H., Kim J.H., Park Y.J., Lee B.M., Kang H.W. Genome-wide identification of pathogenicity genes in Xanthomonas oryzae pv. oryzae by transposon mutagenesis. Plant Pathol. 2008;57:1136–1145. [Google Scholar]
- 130.McCarthy Y., Ryan R.P., O'Donovan K., He Y.Q., Jiang B.L., Feng J.X., Tang J.L., Dow J.M. The role of PilZ domain proteins in the virulence of Xanthomonas campestris pv. campestris. Mol. Plant Pathol. 2008;9:819–824. doi: 10.1111/j.1364-3703.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ojanen-Reuhs T., Kalkkinen N., Westerlund-Wikstrom B., van Doorn J., Haahtela K., Nurmiaho-Lassila E.L., Wengelnik K., Bonas U., Korhonen T.K. Characterization of the fimA gene encoding bundle-forming fimbriae of the plant pathogen Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 1997;179:1280–1290. doi: 10.1128/jb.179.4.1280-1290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vandoorn J., Boonekamp P.M., Oudega B. Partial characterization of fimbriae of Xanthomonas campestris pv. hyacinthi. Mol. Plant Microbe Interact. 1994;7:334–344. doi: 10.1094/mpmi-7-0334. [DOI] [PubMed] [Google Scholar]
- 133.Wang L., Makino S., Subedee A., Bogdanove A.J. Novel candidate virulence factors in rice pathogen Xanthomonas oryzae pv. oryzicola as revealed by mutational analysis. Appl. Environ. Microbiol. 2007;73:8023–8027. doi: 10.1128/AEM.01414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schaad N.W., Jones J.B., Chun W. Laboratory Guide of Identification of Plant Pathogenic Bacteria. APS Press; St. Paul, MN, USA: 2001. [Google Scholar]
- 135.Romantschuk M., Bamford D.H. The causal agent of halo blight in bean, Pseudomonas syringae pv. phaseolicola, attaches to stomata via its pili. Microb. Pathog. 1986;1:139–148. doi: 10.1016/0882-4010(86)90016-1. [DOI] [PubMed] [Google Scholar]
- 136.Suoniemi A., Bjorklof K., Haahtela K., Romantschuk M. Pili of Pseudomonas syringae pathovar syringae enhance initiation of bacterial epiphytic colonization of bean. Microbiol. SGM. 1995;141:497–503. [Google Scholar]
- 137.Roine E., Raineri D.M., Romantschuk M., Wilson M., Nunn D.N. Characterization of type IV pilus genes in Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 1998;11:1048–1056. doi: 10.1094/MPMI.1998.11.11.1048. [DOI] [PubMed] [Google Scholar]
- 138.Taguchi F., Ichinose Y. Role of type IV pili in virulence of Pseudomonas syringae pv. tabaci 6605: Correlation of motility, multidrug resistance and HR-inducing activity to a nonhost plant. Mol. Plant Microbe Interact. 2011;24:1001–1011. doi: 10.1094/MPMI-02-11-0026. [DOI] [PubMed] [Google Scholar]
- 139.Alfano J.R., Collmer A. Bacterial pathogens in plants: Life up against the wall. Plant Cell. 1996;8:1683–1698. doi: 10.1105/tpc.8.10.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ZIP-Document (ZIP, 15163 KB)