Abstract
Balance and gait impairments characterize progression of Parkinson’s disease (PD), predict fall risk, and are important contributors to reduced quality of life. Advances in technology of small, body-worn inertial sensors have made it possible to develop quick, objective measures of balance and gait impairments in the clinic for research trials and clinical practice. Objective balance and gait metrics may eventually provide useful biomarkers for PD. In fact, objective balance and gait measures are already being used as surrogate end-points for demonstrating clinical efficacy of new treatments, in place of counting falls from diaries, using stop-watch measures of gait speed, or clinical balance rating scales. This review summarizes the types of objective measures available from body-worn sensors. We organize the metrics based on the neural control system for mobility affected by PD: postural stability in stance, postural responses, gait initiation, gait (temporal-spatial lower and upper body coordination and dynamic equilibrium), postural transitions, and freezing of gait. However, the explosion of metrics derived by wearable sensors during prescribed balance and gait tasks that are abnormal in people with PD do not yet qualify as behavioral biomarkers because many balance and gait impairments observed in PD are not specific to the disease, nor shown to be related to specific pathophysiologic biomarkers. In the future, the most useful balance and gait biomarkers for PD will be those that are sensitive and specific for early PD and related to the underlying disease process.
Keywords: balance, gait, technology, clinical trials
Introduction
Balance and gait disorders in PD
The biological control of balance and gait has particular relevance for PD because mobility disability is an inevitable consequence of the disease and one of the most important mediators of quality of life (1). Balance refers to control of the body center of mass during daily activities such as standing, getting out of a chair, turning, as well as recovering equilibrium in response to external postural perturbations (2). Gait is the result of control of locomotion (progression of the body with rhythmical coordination of all 4 limbs) combined with control of dynamic equilibrium (ie; balance while moving) of the body center of mass (3). Functional mobility requires quick, flexible changes in balance and gait strategies with changing conditions and demands, and this agility aspect of balance and gait control is affected by PD, as well many aspects of balance and gait (4). Although there is an exponentially increasing literature quantifying balance and gait disorders in PD, these measures have rarely been used as biomarkers for clinical studies or clinical practice (5).
Why do we need balance and gait biomarkers?
Biomarkers are characteristics that can be measured as an indicator of a biological process. Biomarkers serve several important purposes: 1) to provide surrogate end-points for demonstrating clinical efficacy of new treatments, such as neuroprotective therapies; 2) help stratify heterogeneous PD phenotypes and 3) help diagnose symptomatic and pre-symptomatic disease (6). The best biomarkers are linked to fundamental features of PD neuropathology, are able to monitor disease status, are correlated to clinical disease progression, and may be sensitive to preclinical disease. An ideal biomarker of neurodegeneration should be inexpensive, non-invasive, simple to use and scientifically sound (e.g. valid, reliable, and sensitive to change) (6).
Balance and gait biomarkers are potentially valuable as surrogate end-points to reduce the size and length of clinical trials focused on mobility. Currently, these trials depend primarily upon rating scales like the Unified Parkinson’s Disease Rating Scale; infrequent events, such as falls; or upon subjective reports, such as diaries or questionnaires, to determine changes in mobility. Objective balance and gait biomarkers could also be helpful in clinical practice to monitor effects of interventions and prognosis. Biomarkers of balance control could be especially useful to monitor non-dopaminergic degeneration in PD because several types of balance control may not improve, or may even worsen, with levodopa or deep brain stimulation (7–9).
Wearable technology and Biomarkers
To be useful for clinicians, objective measures of balance and gait need to be available outside the laboratory and recent advances in body-worn sensors have recently made this portability possible. Laboratory tests of gait and balance involve expensive, highly technical, non-portable equipment such as video-based motion analysis systems and force plates that are not practical for clinical environments or for multisite clinical trials. Laboratory data analysis is also quite time-consuming and labor intensive so it is not practical for large, clinical trials. Gait mats that can be rolled out on a corridor solve the problem of automatic, quick analysis of gait but their bulkiness, expense, and lack of upper body measurement makes them impractical and/or insensitive to balance disorders and early markers of PD (10, 11). Recently, body-worn sensors consisting of accelerometers, gyroscopes, footswitches and/or insole pressure sensors can quickly and inexpensively provide accurate measures of balance and gait for clinical environments. (12–15).
Objective metrics have been developed to target different types of balance and gait impairments affecting a variety of neural control systems associated with PD. Table 1 summarizes the most common balance and gait kinematic metrics from body-worn sensors during prescribed motor tasks. Each of the metrics in Table 1 were first shown to be sensitive to PD in laboratory studies using 3-D motion analysis, forceplates, EMG and other time-consuming approaches impractical for clinical biomarkers. Recent technological advances in portable, body-worn sensors have provided the infrastructure to translate these metrics into balance and gait biomarkers practical for large numbers of patients in busy clinics (16).
Table 1.
Examples of objective balance and gait metrics sensitive to PD that could provide biomarkers grouped by gait and balance impairments.
| Neural Control Systems | Impairments | Task | Metrics from body-worn sensors |
|---|---|---|---|
| I. Postural Sway | Postural Instability | Quiet stance (≥30s) | Sway Velocity, Sway Area, Sway jerkiness,(25, 28, 29), Sway frequency (24) |
| II. Postural Responses | Ineffective stepping response | Push and Release | Latency, Step length, Number of steps, Time to Equilibrium (38) |
| III. Anticipatory Postural Adjustments (APA) | Impaired gait Initiation | Step Initiation | Lateral and Sagittal Peak APA (39) |
| IV. Gait | |||
| Dynamic Equilibrium | Gait Instability | Straight Walking at prefered gait speed | Stride Time Variability (14, 15, 58), Double Support Time (10, 14) |
| Upper Body Control | “En-bloc” | Peak arm velocity, Trunk Rotation (10) | |
| Spatio-temporal Coordination | Slow gait | Gait Velocity, Cadence, Stride Length (10, 48, 63) | |
| V. Postural Transitions | Difficulty changing motor programs | Timed-Up and Go | Turning Duration (10, 62, 63), Turning Jerk (62), Range and-to-Stand and Sit Stand-to-Sit Jerk (63) |
| VI. Unknown (66) | Freezing of Gait | Turn 360° degrees | Shank Frequency Ratio (65, 66, 68) |
This review will summarize how new, wearable technologies can provide valuable, objective measures of PD balance and gait disorders from Table 1, as candidates for behavioral biomarkers in clinical trials and clinical practice. We also suggest how potential balance and gait biomarkers should be validated to understand their uses and limitations.
I. Postural Sway in Stance
Postural sway is a sensitive measure of the complex sensorimotor control loop responsible for control of standing balance so it provides an excellent measure of postural instability (17, 18). Postural sway can be characterized by several, independent metrics: area, velocity, frequency, and jerk (19, 20). Sway area, velocity and frequency are larger in elderly people prone to falls compared to a younger population or elderly with no falls (21–23). Traditionally, postural sway has been measured with a forceplate under the feet, but recently, small 2-axis accelerometers attached to the pelvis, near the body center of mass, have been used to provide similar information about body sway (24–27). The use of accelerometers to measure body sway makes it practical for clinical and even home environments.
Figure 1 shows postural sway measured with an accelerometer on the pelvis. One of the most discriminative measures between untreated PD and healthy-matched controls has been found to be jerk of postural sway (e.g.; derivative of trunk acceleration). y Postural sway during quiet stance with eyes open is less smooth in patients with untreated mild-to-moderate PD who have never taken levodopa compared to controls (28). Untreated PD also showed a higher sway dispersion (measured by RMS of trunk acceleration) and mean sway velocity compared to controls (24). In addition, a recent study suggested that abnormal postural sway could be used to identify people at high risk for developing PD (presence of an enlarged area of hyperechogenicity of the substantia nigra, and the additional occurrence of one PD cardinal motor signs or risk factors) (29). Specifically, people at high risk for developing PD showed higher sway dispersion (measured by RMS of acceleration) and decreased sway jerk (differentiation of acceleration) compared to PD and healthy controls, but only when in semi-tandem stance on foam with eyes-closed (29). Sway dispersion and sway velocity may also be related to progression of PD (30). The effect of DBS reduces sway area whereas levodopa increases postural sway area, suggesting that DBS acts on postural control through a different mechanism than levodopa circuitry (31).
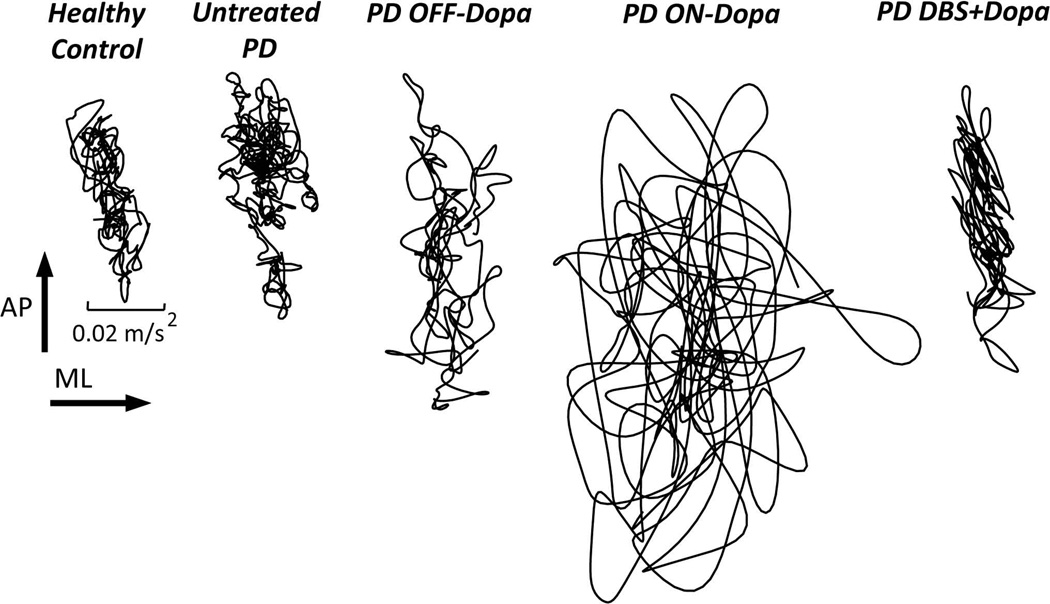
Figure 1.
Stabilogram from pelvis acceleration traces (lateral versus anteroposterior) during 30s of quiet stance, eyes open, normal stance, from a representative healthy control, untreated patient with early PD, moderate PD patient OFF and ON medication before DBS surgery, and 6 months after bilateral DBS surgery in STN (from left to right). Note that sway area is larger than normal, even in early, untreated PD, and increases with levodopa but decreases with DBS in STN with additive effects of both levodopa and DBS. Stabilograms from center of pressure excursions of a forceplate result in similar metrics of sway in quiet stance (25,27).
Postural sway may be a good overall measure of “balance control” that can be used as a primary outcome for interventions. For example, we found that postural sway measures were more sensitive to a physical therapy postural agility program than clinical rating scales (32). However, to be most useful, we will need to relate postural sway to patient-centered outcomes such as reduction of falls in PD (22, 33).
II. Postural Responses
Automatic postural responses to external perturbations are educed in amplitude, albeit with normal onset latencies, in patients with PD (7). Both feet-in-place postural responses and corrective stepping responses show this bradykinesia of postural responses in people with PD (34). In fact, these weak postural responses are responsible for the small, repeated backward steps (retropulsion) to a backward pull on the shoulders in patients with PD (35).
Laboratory studies have shown that PD, specifically, also affects the ability to flexibly adapt postural response strategies when the initial conditions change (2, 36). For example, when postural perturbations change from a translation to a rotation or when support conditions change from holding a stable support to no support, subjects without PD immediately modify their postural responses to take into account the new physical constraints of the situation. In contrast, patients with PD gradually adapt their responses with trial and error, over several trials (37). These laboratory protocols highlighting basal ganglia-specific set-switching control mechanisms have yet to be translated into practical biomarkers. For this reason, our laboratory is now instrumenting the backward stepping responses using inertial sensors on the trunk and ankles to obtain postural response latencies, size and number of the corrective steps, as well as time to recover equilibrium (38).
III. Anticipatory Postural Adjustments for Step Initiation
Step initiation is preceded by anticipatory postural adjustments (APAs) that shift the body off the stepping leg and forward in front of the base of foot support. Like sway, these postural adjustments have traditionally been measured with a displacement of the center of pressure on a forceplate under the feet. However, recently, studies have demonstrated how well accelerometers on the pelvis could be used to detect and measure the amplitude of APAs as the amount of force used to move the body center of mass is proportional to the acceleration of the center of mass in the opposite direction (39–41). APAs are well known to be too small in patients with PD (42). Of course, bradykinetic APAs are associated with bradykinetic gait so small APAs are associated with small step length and slow walking speed and delayed onset of stepping (43, 44). Figure 2 illustrates the small lateral APA prior to step initiation in a representative untreated PD and control subject.
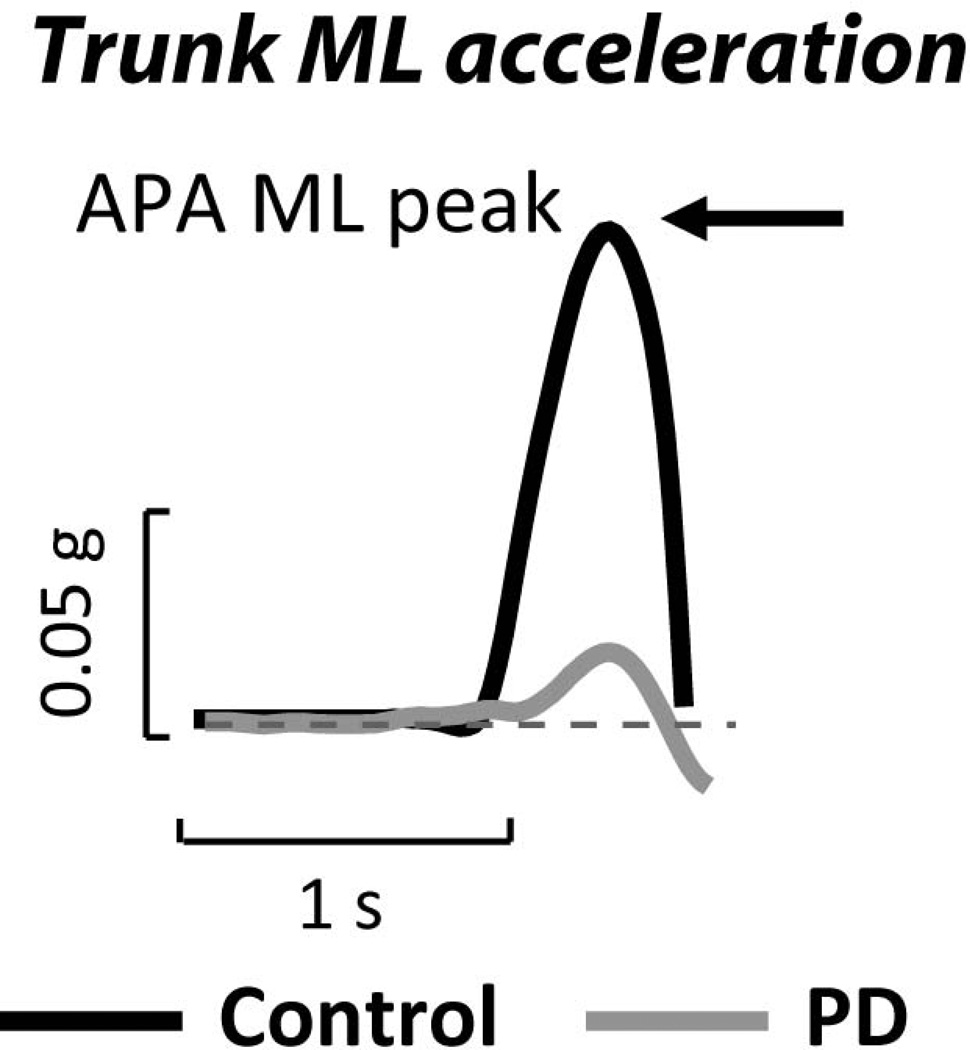
Figure 2.
Anticipatory postural adjustment (APA) as measured from pelvis lateral acceleration in a representative healthy control and patient with early, untreated PD prior to taking a step. APAs are smaller than normal early in progression of PD, are improved with levodopa but worsened by DBS (8). APAs from center of pressure excursion of a forceplate result in similar APA metrics (39).
IV. Gait: Spatial-temporal Coordination, Upper body Control and Dynamic Equilibrium
Gait is a complex sensorimotor activity that involves spatial-temporal coordination of the legs, coordination of the trunk and arms, as well as dynamic equilibrium, all of which are affected by PD (3, 4).
Spatial-temporal Coordination
People with PD have been shown to have significantly slower gait, with less foot clearance and smaller step lengths (14, 45). However, not all spatial-temporal characteristics of gait are linearly related to severity of disease, and few are specific to PD (14, 46, 47). For example, cadence (steps per minute) has been shown to be slow in untreated PD (10) but then faster than normal with freezing of gait or to compensate for short strides (48). The spatial and temporal characteristics of leg movements during gait have traditionally required video-based motion analysis or gait mats. However, most of the same spatial and temporal characteristics of gait can be obtained with body-worn inertial sensors and have been validated with laboratory optical, motion analysis gold-standards (49). Only stride width is difficult to obtain with body-worn sensors.
Upper Body Control
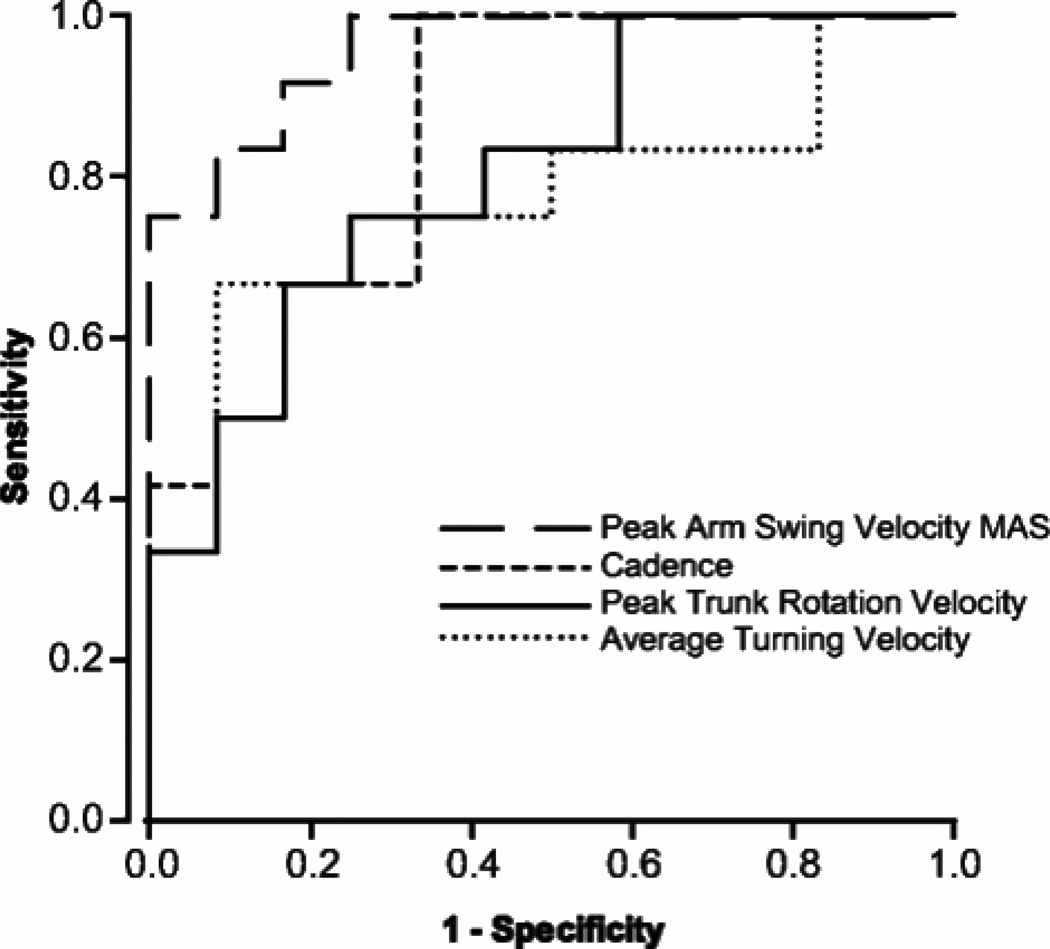
Although PD is well known to be associated with a slow gait and short step length, it has been recently shown that the most sensitive metrics of gait in early PD are not directly from the legs but are from the upper body during walking. For example, we found that reduced trunk rotation (‘En-bloc’) while walking and reduced arm swing were the most sensitive and specific (>0.9) early signs of gait impairment in a study of 24 early, untreated, idiopathic PD subjects (10). Fig. 3 shows sensitivity/specificity of the 4 best metrics from the ITUG to separate untreated people with early PD from healthy control subjects the same age (10). Our preliminary data suggests that arm swing, but not leg movements, also show a significant reduction across 18 months in de-novo patients with PD.
Figure 3.
A. Receiver Operating Characteristics (ROC) curves for the ITUG parameters (out of 52 parameters measured) that best discriminate between healthy control subjects and untreated PD subjects from Zampieri et al., (10): peak arm velocity of the more affected side (MAS), cadence, peak trunk rotation velocity (yaw), and average turning velocity during 180 degree turn during gait (Adapted from Zampieri et al., JNNP 2010).
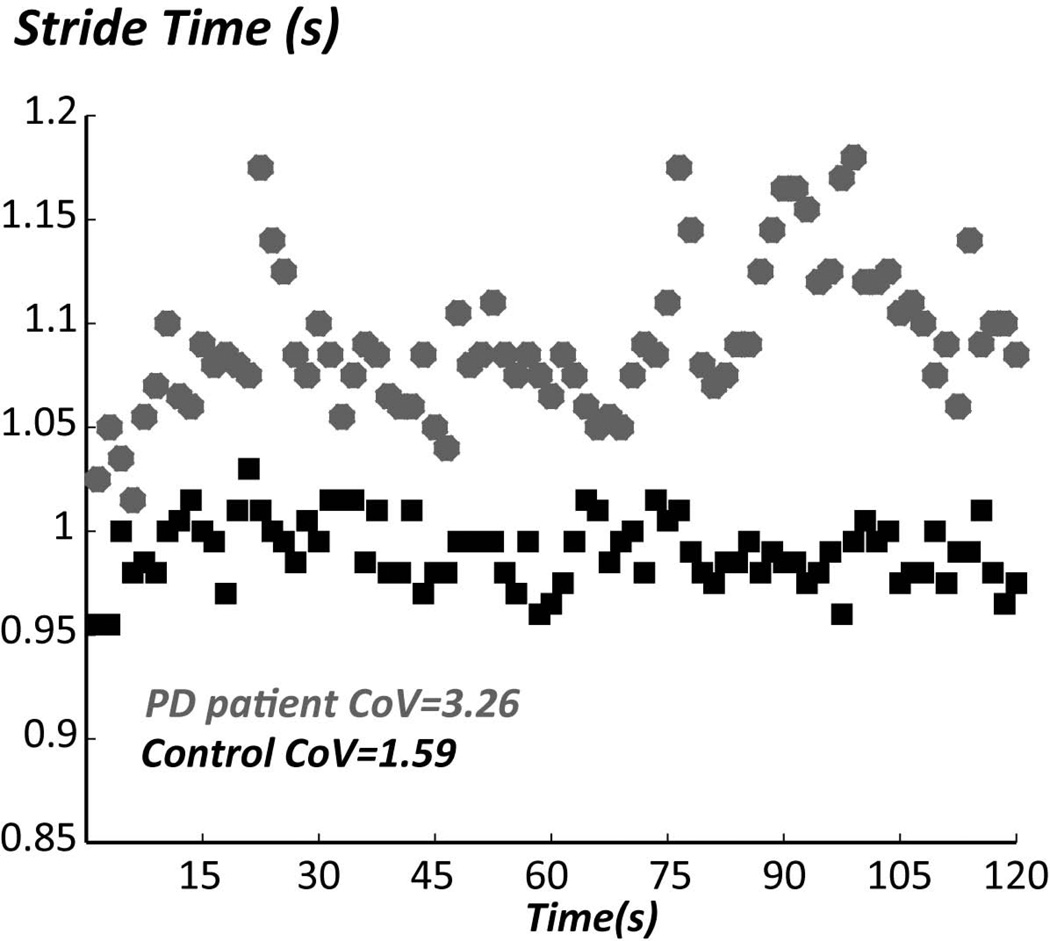
B. Stride time (extracted from angular velocities of the lower legs) for each stride (consisting of a left plus right step duration) during a 2-minute walk in a representative patient with PD OFF medication and in an age-matched healthy control subject. The coefficient of variation (CoV) is the standard deviation/mean of stride times for the 2-minute walk.
Dynamic Equilibrium
Some gait measures are particularly related to dynamic equilibrium control, that is, controlling the body center of mass while moving the base of foot support. Equilibrium during gait is maintained by trunk and hip muscle control of lateral trunk motion and by lateral compensatory postural stepping responses (3, 50, 51). To monitor equilibrium control during gait, body-worn sensors can measure extent of lateral trunk displacement, duration of double support time, stride time or stride width variability, all which predict falling as they get larger (52–54). How and why gait variability is related to falling is controversial but may be due to abnormal timing of central pattern generators or to increase in compensatory foot placements to control poor balance while walking (14, 15, 50, 55). Patients with PD show increased time spent in double support phase of gait as well as increased stride time variability ((14, 15) Fig. 3B). Levodopa reduces stride time variability, decreases double support time and increases gait speed (56, 57), all consistent with improvements in dynamic equilibirum during gait. DBS in the subthalamic nucleus can also reduce stride time variability and improves gait speed, although DBS in the subthalamic nucleus worsens postural stepping responses and anticipatory postural adjustment for step initation (8, 9). In addition, a recent paper from Mirelmann et al., found gait variability to be impaired in a population with increased risk of developing PD (healthy carriers of the LRRK2 G2019S mutation), especially when dual-tasking or when walking at a fast speed (58).
V. Postural Transitions
Postural transitions include changes in postures, such as sit-to-stand or stand-to-sit or a change in direction of walking (ie; turning). These postural transitions seem to be particularly affected by PD and predict risk of falls in the elderly (59–61). In fact, the duration of the popular Timed Up and Go test (TUG), involving standing up from a chair, walking 3 meters, turning 180 degree and returning to sit in the chair, has been shown to separate fallers and nonfallers with PD or the elderly (62–64). Recently, the TUG has been instrumented with inertial sensors (ITUG) to provide objective measures of postural transitions as well as of gait, although it is often extended from a 3-meter to a 7-meter walking distance (10, 62, 63).
During the ITUG, the speed of trunk rotation during turning and the duration of the sit-to-stand and stand-to-sit transitions are very sensitive to early, untreated PD (10). A new study showed that a combination of measures from sit-to-stand and turning (RMS of sit-to-stand trunk acceleration, vertical and mediolateral jerk of turning) best discriminates between early PD tested OFF medication and control subjects during an ITUG (62). Another study found that jerk during the Sit-to-Stand and Stand-to-Sit transitions best discriminates between PD subjects and controls although the groups had similar TUG durations (63). ‘Jerk’ represents a change in acceleration of motion, and tends to be minimized in normal movement coordination, with the possibility that the basal ganglia plays a role in this coordination.
VI. Freezing of Gait
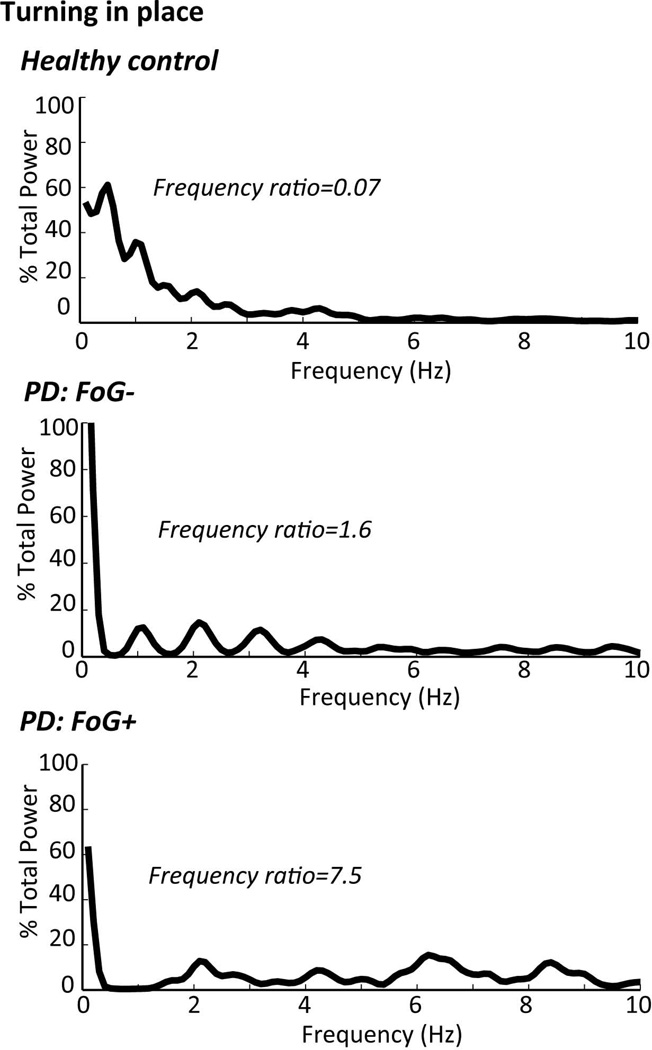
Freezing of gait may also be identified and quantified by its characteristic frequencies of shank motion during “trembling of the knees” as patients attempt to step when they feel their feet are glued to the floor (65–67). For example, Fig. 4 illustrates how a ratio of the power in the freezing frequencies (3–8 Hz) divided by the power in the gait frequencies (0.5–3 Hz) of horizontal shank acceleration can be used to provide an objective ‘freezing ratio’ (68). In this example, the freezing ratio was calculated when the subjects were turning 360 degrees in place, but similar freezing ratios can be calculated during gait or more complex tasks such as the Timed Up and Go task (68). However, some forms of freezing may not include “trembling of the knees” and it is not clear if this approach will work to identify brief freezing episodes during continuous monitoring of gait.
Figure 4.
Power Spectral Densities (PSD) and Frequency Ratios of anteroposterior shank acceleration during a 2-min 360° turn-in-place. The PSD shows that most of the power of leg motion during healthy turning is below 2 Hz, with more high frequencies represented in patients with PD, especially in patients with PD who have freezing of gait (FoG+), The Frequency rations is the power at 3–8Hz/ power at 0.5–3Hz). The higher frequencies represent “trembling in place” and the lower frequencies the stepping movements. From the top to bottom: healthy control subject, a PD subject with freezing of gait OFF medication and a PD subject with similar Motor UPDRS scores, without freezing of gait OFF medication.
Future Directions
One challenge for using body-worn inertial sensors to obtain balance and gait biomarkers is the availability of commercial systems that are easy to use and interpret, although this challenge is quickly being met. However, it is not clear which specific protocol or metrics should be used to measure PD-sensitive and specific balance and gait behaviors, although protocols that include testing a variety of neural control systems for mobility, such as the Timed Up and Go and the Stand and Walk test are sure to be the most valuable as they measure several, independent aspects of balance and gait (10, 69). In fact, different metrics, and even different protocols may be necessary for patients with other types of movement disorders than idiopathic PD. For example, cerebellar ataxia and vascular PD may be characterized by excessive lateral trunk motion and wide-based gait so upper body motions and distance between feet must be measured.
It is important to consider, however, that even a simple balance or gait protocol such as the Timed Up and Go test can result in over 100 objective metrics and it is not always obvious which, specific metrics provide the best biomarkers and which are independent of each other. One approach to this redundancy problem is to combine metrics into a meaningful “Combined Mobility Score” but the disadvantage of a score made from combining metrics is in understanding which specific aspects of balance and gait it represents. For example, a score based on improvement with levodopa will not reflect postural responses that worsen with levodopa (36). In fact, combinations of specific metrics of balance and gait may be needed to fully characterize subtypes of mobility problems underlying a variety of parkinsonism and Parkinson-plus syndromes and to determine which, specific postural circuits can be changed with intervention and which are resistant to change.
Current studies are examining the value of obtaining continuous measures of mobility during daily activities with body-worn sensors (70–72). Accelerometers on the legs and trunk can identify when subjects are sitting, lying and standing or walking (and their walking cadence) as a measure of their activity level. For example, a recent study showed reduced amount and intensity of walking bouts in the community of PD subjects after a year (70). Another study showed a significant increase in length and variability of walking bouts after subthalamic nucleus DBS compared to pre-surgery, perhaps suggesting an increased diversity of walking pattern and flexibility (71). More recently, frequency-derived measures from one accelerometer on the trunk are valid and sensitive estimates of stride-to-stride variability to assess gait “quality” (and not only quantity) in real-life settings in PD (72). Identifying mobility in real world environments is certainly more ecological than occasional measures when being observed in the clinic. However, it would be even more useful for PD to fully characterize the quality of walking in the community, how mobility fluctuates during the day and with the medication cycle. It would also be useful to characterize challenges of postural transitions such as turning and sit to stand activities.
To promote objective measures of balance and gait into useful biomarkers, future studies should relate these metrics with other specific, pathophysiological markers from imaging, genetics, blood and other body fluids that relate to the pathophysiology of PD. However, there is still poor understanding of the specific neural circuits involved in each aspect of balance and gait control (73). New insights into the neuroanatomy and neurophysiology of control of balance and gait can come from developing PD phenotypes based on specific impairments of balance and gait must related to specific underlying mechanisms of the neurodegenerative process (eg; see (74)). Most balance and gait behaviors currently associated with PD, such as slowed and variable gait and increased body sway are not specific for this disease, although they can be useful because they are sensitive, reliable, and surrogates of fall risk. Other measures, such as reduced arm swing, narrow foot placement and freezing characteristics may be more specific for parkinsonism and thus may be more useful for developing posture and gait phenotypes.
Summary
Body-worn sensors have provided objective metrics of balance and gait that are quick and easy to use. As potential biomarkers, many of these metrics have already been shown to be: 1) valid relative to laboratory gold-standards, 2) related to clinical gold standards of severity such as the UPDRS, 3) sensitive to early disease, 4) reliable, and 5) responsive to Levodopa, deep brain stimulation and physical therapy intervention. Since any specific metric does not reflect all of the domains of balance and gait affected by PD, a protocol that includes several different aspects of mobility and a score that combines metrics among these aspects needs as a potential biomarkers. However, a major gap in developing gait and balance biomarkers is identifying behavioral measures related to PD-specific pathology and in identifying preclinical measures from longitudinal studies in people at risk for developing PD. The most useful gait biomarkers would be those that can aid in the diagnosis of presymptomatic PD to document potential neuroprotective interventions. The most promising measures seem to be related to reduced arm swing, reduced trunk motion during gait, abnormal postural sway, reduced anticipatory postural adjustments during postural transitions, and gait variability (10, 29, 39, 58). However, larger, longitudinal cohorts are needed to assess the sensitivity, specificity and validity of these potential biomarkers for PD.
Acknowledgments
Funding sources for the study: Support from the National Institutes on Aging, the National Center of Medical Rehabilitation Research and the Kinetics Foundation, and OHSU.
Full Financial Disclosure (for the preceding 12 months)
Fay Horak has the following research support: R37 -NIH/NIA MERIT - Peripheral & Central Postural Disorders in the Elderly, 1R41 -STTR from NINDS - Continuous Monitoring of Turning in Patients with Parkinson's Disease, 1 R41 - STTR from NIH/CHHD/NCMRR - A Short Instrumented Test of Mobility for Neurological Disorders, 1 R01 -NIH/NCI -Preventing Falls After Cancer: Tai Chi vs. Strength Training, The Parkinson Alliance - Instrumented Balance and Gait Measures to Evaluate Effects of DBS Surgery, National Multiple Sclerosis Society -Rehabilitation Research Training in Postural Control of Multiple Sclerosis.
Relevant conflicts of interest/financial disclosures: Dr. Horak and OHSU have significant financial interests in APDM, a company that might have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU and the Integrity Oversight Council.
Martina Mancini post-doctoral work has been funded by a NIH/NINDS Challenge Grant (RC1 NS068678 Horak (PI) until September 2011), and NIH/NIA Merit Award (R37, AG006457, Horak PI, from September 2011 to March 2012); currently funded by Continuous monitoring of Turning in Patients with Parkinson’s disease (R41 NS07608801-01 Horak (co-PI) and Feasibility of relating continuous monitoring of turning mobility to falls and executive function (ORCATECH Pilot Grant, Mancini PI).
Footnotes
- Research project: A. Conception, Horak. B. Organization, Horak B. C. Execution, Horak and Mancini.
- Statistical Analysis, N/A.
- Manuscript: A. Writing of the first draft, Horak. B. Review and critique, Horak, Mancini.
References
- 1.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 2.MacPherson JMHF. Posture. Chapter 41. In: Kandel ERSJ, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of Neural Science. Fifth edition. New York, NY: McGraw-Hill; 2012. [Google Scholar]
- 3.Winter D. Biomechanics and Motor Control of Human Movement. Fourth Edition ed. Hoboken, New Jersey: John Wiley & Sons, Inc; 2009. [Google Scholar]
- 4.Schoneburg B, Mancini M, Horak F, Nutt JG. Framework for understanding balance dysfunction in Parkinson's disease. Movement Disorders. 2013 doi: 10.1002/mds.25613. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutt JG, Horak FB, Bloem BR. Milestones in gait, balance, and falling. Mov Disord. 2011;26(6):1166–1174. doi: 10.1002/mds.23588. [DOI] [PubMed] [Google Scholar]
- 6.Gerlach M, Maetzler W, Broich K, Hampel H, Rems L, Reum T, et al. Biomarker candidates of neurodegeneration in Parkinson's disease for the evaluation of disease-modifying therapeutics. J Neural Transm. 2012;119(1):39–52. doi: 10.1007/s00702-011-0682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75(6):2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 8.Rocchi L, Carlson-Kuhta P, Chiari L, Burchiel KJ, Hogarth P, Horak FB. Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in Parkinson disease: laboratory investigation. J Neurosurg. 2012;117(6):1141–1149. doi: 10.3171/2012.8.JNS112006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St George RJ, Carlson-Kuhta P, Burchiel KJ, Hogarth P, Frank N, Horak FB. The effects of subthalamic and pallidal deep brain stimulation on postural responses in patients with Parkinson disease. J Neurosurg. 2012;116(6):1347–1356. doi: 10.3171/2012.2.JNS11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81(2):171–176. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17(1):68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 12.Mancini M, King L, Salarian A, Holmstrom L, McNames J. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J Bioengineer & Biomedical Sci S. 2012;1:2. doi: 10.4172/2155-9538.S1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson's disease. Eur J Neurosci. 2006;24(6):1815–1820. doi: 10.1111/j.1460-9568.2006.05033.x. [DOI] [PubMed] [Google Scholar]
- 14.Hausdorff JM. Gait dynamics in Parkinson's disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19(2):026113. doi: 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson's disease and Huntington's disease. Mov Disord. 1998;13(3):428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- 16.Shany T, Redmond SJ, Marschollek M, Lovell NH. Assessing fall risk using wearable sensors: a practical discussion. A review of the practicalities and challenges associated with the use of wearable sensors for quantification of fall risk in older people. Z Gerontol Geriatr. 2012;45(8):694–706. doi: 10.1007/s00391-012-0407-2. [DOI] [PubMed] [Google Scholar]
- 17.Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res. 1995;105(1):101–110. doi: 10.1007/BF00242186. [DOI] [PubMed] [Google Scholar]
- 18.Maurer C, Mergner T, Peterka RJ. Multisensory control of human upright stance. Exp Brain Res. 2006;171(2):231–250. doi: 10.1007/s00221-005-0256-y. [DOI] [PubMed] [Google Scholar]
- 19.Maurer C, Peterka RJ. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J Neurophysiol. 2005;93(1):189–200. doi: 10.1152/jn.00221.2004. [DOI] [PubMed] [Google Scholar]
- 20.Rocchi L, Chiari L, Cappello A. Feature selection of stabilometric parameters based on principal component analysis. Med Biol Eng Comput. 2004;42(1):71–79. doi: 10.1007/BF02351013. [DOI] [PubMed] [Google Scholar]
- 21.Merlo A, Zemp D, Zanda E, Rocchi S, Meroni F, Tettamanti M, et al. Postural stability and history of falls in cognitively able older adults: the Canton Ticino study. Gait Posture. 2012;36(4):662–666. doi: 10.1016/j.gaitpost.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Melzer I, Kurz I, Oddsson LI. A retrospective analysis of balance control parameters in elderly fallers and non-fallers. Clin Biomech (Bristol, Avon) 2010;25(10):984–988. doi: 10.1016/j.clinbiomech.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Norris JA, Marsh AP, Smith IJ, Kohut RI, Miller ME. Ability of static and statistical mechanics posturographic measures to distinguish between age and fall risk. J Biomech. 2005;38(6):1263–1272. doi: 10.1016/j.jbiomech.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Palmerini L, Rocchi L, Mellone S, Valzania F, Chiari L. Feature selection for accelerometer-based posture analysis in Parkinson's disease. IEEE Trans Inf Technol Biomed. 2011;15(3):481–490. doi: 10.1109/TITB.2011.2107916. [DOI] [PubMed] [Google Scholar]
- 25.Mancini M, Salarian A, Carlson-Kuhta P, Zampieri C, King L, Chiari L, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. doi: 10.1186/1743-0003-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Sullivan M, Blake C, Cunningham C, Boyle G, Finucane C. Correlation of accelerometry with clinical balance tests in older fallers and non-fallers. Age Ageing. 2009;38(3):308–313. doi: 10.1093/ageing/afp009. [DOI] [PubMed] [Google Scholar]
- 27.Whitney SL, Roche JL, Marchetti GF, Lin CC, Steed DP, Furman GR, et al. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: a measure of balance. Gait Posture. 2011;33(4):594–599. doi: 10.1016/j.gaitpost.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancini M, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Chiari L. Trunk accelerometry reveals postural instability in untreated Parkinson's disease. Parkinsonism Relat Disord. 2011;17(7):557–562. doi: 10.1016/j.parkreldis.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maetzler W, Mancini M, Liepelt-Scarfone I, Muller K, Becker C, van Lummel RC, et al. Impaired trunk stability in individuals at high risk for Parkinson's disease. PLoS One. 2012;7(3):e32240. doi: 10.1371/journal.pone.0032240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson's disease: a pilot longitudinal study. Gait Posture. 2012;36(3):471–476. doi: 10.1016/j.gaitpost.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73(3):267–274. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King LA, Salarian A, Mancini M, Priest KC, Nutt J, Serdar A, et al. Exploring outcome measures for exercise intervention in people with Parkinson’s disease. Parkinson's Disease. 2013 doi: 10.1155/2013/572134. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melzer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and non-fallers. Age Ageing. 2004;33(6):602–607. doi: 10.1093/ageing/afh218. [DOI] [PubMed] [Google Scholar]
- 34.King LA, St George RJ, Carlson-Kuhta P, Nutt JG, Horak FB. Preparation for compensatory forward stepping in Parkinson's disease. Arch Phys Med Rehabil. 2010;91(9):1332–1338. doi: 10.1016/j.apmr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs JV, Horak FB, Van Tran K, Nutt JG. An alternative clinical postural stability test for patients with Parkinson's disease. J Neurol. 2006;253(11):1404–1413. doi: 10.1007/s00415-006-0224-x. [DOI] [PubMed] [Google Scholar]
- 36.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111(1):46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 37.Chong RK, Horak FB, Woollacott MH. Time-dependent influence of sensorimotor set on automatic responses in perturbed stance. Exp Brain Res. 1999;124(4):513–519. doi: 10.1007/s002210050647. [DOI] [PubMed] [Google Scholar]
- 38.El-Gohary M, Smith B, Carlson-Kuhta P, Horak F. Society for Neuroscience. San Diego, CA: 2013. Instrumented Push and Release Test (IPUSH) for postural responses using wearable inertial sensors. [Google Scholar]
- 39.Mancini M, Zampieri C, Carlson-Kuhta P, Chiari L, Horak FB. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson's disease: an accelerometer-based approach. Eur J Neurol. 2009;16(9):1028–1034. doi: 10.1111/j.1468-1331.2009.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Mendez R, Sekine M, Tamura T. Detection of anticipatory postural adjustments prior to gait initiation using inertial wearable sensors. J Neuroeng Rehabil. 2011;8:17. doi: 10.1186/1743-0003-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocchi L, Mancini M, Chiari L, Cappello A. Dependence of anticipatory postural adjustments for step initiation on task movement features: a study based on dynamometric and accelerometric data. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1489–1492. doi: 10.1109/IEMBS.2006.260731. [DOI] [PubMed] [Google Scholar]
- 42.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12(2):206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 43.Breniere JDM, Bouisset S. Are dynamic phenomena prior to stepping essential to walking? J Mot Behav. 1987;19:62–76. doi: 10.1080/00222895.1987.10735400. [DOI] [PubMed] [Google Scholar]
- 44.Breniere Y, Do MC. Control of gait initiation. J Mot Behav. 1991;23(4):235–240. doi: 10.1080/00222895.1991.9942034. [DOI] [PubMed] [Google Scholar]
- 45.Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain. 1994;117(Pt 5):1169–1181. doi: 10.1093/brain/117.5.1169. [DOI] [PubMed] [Google Scholar]
- 46.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78(3):278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 47.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 48.Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson's disease and the stride length sequence effect interaction. Brain. 2009;132(Pt 8):2151–2160. doi: 10.1093/brain/awp053. [DOI] [PubMed] [Google Scholar]
- 49.Salarian A, Russmann H, Vingerhoets FJ, Dehollain C, Blanc Y, Burkhard PR, et al. Gait assessment in Parkinson's disease: toward an ambulatory system for long-term monitoring. IEEE Trans Biomed Eng. 2004;51(8):1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 50.O'Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol. 2009;102(3):1411–1419. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boonsinsukh R, Saengsirisuwan V, Carlson-Kuhta P, Horak FB. A cane improves postural recovery from an unpracticed slip during walking in people with Parkinson disease. Phys Ther. 2012;92(9):1117–1129. doi: 10.2522/ptj.20120036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci. 2007;26(4):555–589. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing. 2011;40(4):481–487. doi: 10.1093/ageing/afr055. [DOI] [PubMed] [Google Scholar]
- 55.Rebula JR, Ojeda LV, Adamczyk PG, Kuo AD. Measurement of foot placement and its variability with inertial sensors. Gait Posture. 2013 doi: 10.1016/j.gaitpost.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bryant MS, Rintala DH, Hou JG, Charness AL, Fernandez AL, Collins RL, et al. Gait variability in Parkinson's disease: influence of walking speed and dopaminergic treatment. Neurol Res. 2011;33(9):959–964. doi: 10.1179/1743132811Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lord S, Baker K, Nieuwboer A, Burn D, Rochester L. Gait variability in Parkinson's disease: an indicator of non-dopaminergic contributors to gait dysfunction? J Neurol. 2011;258(4):566–572. doi: 10.1007/s00415-010-5789-8. [DOI] [PubMed] [Google Scholar]
- 58.Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol. 2011;69(1):193–197. doi: 10.1002/ana.22165. [DOI] [PubMed] [Google Scholar]
- 59.Greene BR, Doheny EP, Walsh C, Cunningham C, Crosby L, Kenny RA. Evaluation of falls risk in community-dwelling older adults using body-worn sensors. Gerontology. 2012;58(5):472–480. doi: 10.1159/000337259. [DOI] [PubMed] [Google Scholar]
- 60.Weiss A, Herman T, Plotnik M, Brozgol M, Giladi N, Hausdorff JM. An instrumented timed up and go: the added value of an accelerometer for identifying fall risk in idiopathic fallers. Physiol Meas. 2011;32(12):2003–2018. doi: 10.1088/0967-3334/32/12/009. [DOI] [PubMed] [Google Scholar]
- 61.King LA, Mancini M, Priest K, Salarian A, Rodrigues-de-Paula F, Horak F. Do clinical scales of balance reflect turning abnormalities in people with Parkinson's disease? J Neurol Phys Ther. 2012;36(1):25–31. doi: 10.1097/NPT.0b013e31824620d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmerini L, Mellone S, Avanzolini G, Valzania F, Chiari L. Quantification of Motor Impairment in Parkinson's Disease Using an Instrumented Timed Up and Go Test. IEEE Trans Neural Syst Rehabil Eng. 2013 doi: 10.1109/TNSRE.2012.2236577. [DOI] [PubMed] [Google Scholar]
- 63.Weiss A, Herman T, Plotnik M, Brozgol M, Maidan I, Giladi N, et al. Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson's disease? Med Eng Phys. 2010;32(2):119–125. doi: 10.1016/j.medengphy.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 64.Adame MR, Al-Jawad A, Romanovas M, Hobert MA, Maetzler W, Moller K, et al. TUG Test Instrumentation for Parkinson's disease patients using Inertial Sensors and Dynamic Time Warping. Biomed Tech (Berl) 2012 [Google Scholar]
- 65.Moore ST, MacDougall HG, Ondo WG. Ambulatory monitoring of freezing of gait in Parkinson's disease. J Neurosci Methods. 2008;167(2):340–348. doi: 10.1016/j.jneumeth.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 66.Hausdorff JM, Balash Y, Giladi N. Time series analysis of leg movements during freezing of gait in Parkinson's disease: akinesia, rhyme or reason? Physica A: Statistical Mechanics and its Applications. 2003;321(3–4):565–570. [Google Scholar]
- 67.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10(8):734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mancini M, Priest KC, Nutt JG, Horak FB. Quantifying freezing of gait in Parkinson's disease during the instrumented timed up and go test. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1198–1201. doi: 10.1109/EMBC.2012.6346151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46(2):239–248. [PMC free article] [PubMed] [Google Scholar]
- 70.Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE. Capturing ambulatory activity decline in Parkinson's disease. J Neurol Phys Ther. 2012;36(2):51–57. doi: 10.1097/NPT.0b013e318254ba7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rochester L, Chastin SF, Lord S, Baker K, Burn DJ. Understanding the impact of deep brain stimulation on ambulatory activity in advanced Parkinson's disease. J Neurol. 2012;259(6):1081–1086. doi: 10.1007/s00415-011-6301-9. [DOI] [PubMed] [Google Scholar]
- 72.Weiss A, Sharifi S, Plotnik M, van Vugt JP, Giladi N, Hausdorff JM. Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabil Neural Repair. 2011;25(9):810–818. doi: 10.1177/1545968311424869. [DOI] [PubMed] [Google Scholar]
- 73.Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39(2):786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 74.Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136(Pt 8):2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]