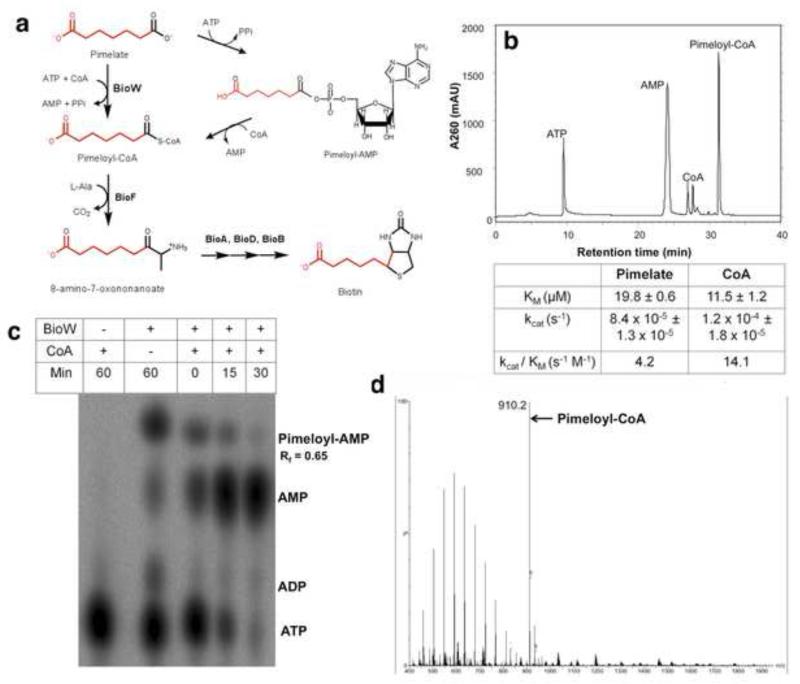
Figure 1. Schematic of B. subtilis biotin biosynthesis and the BioW reaction.
(a) The pathway begins with the conversion of pimelate to pimeloyl-CoA by an ATP-dependent reaction catalyzed by BioW with the adenylate as intermediate. The pimelate thioester provides most the carbon atoms of the biotin as indicated in red. The pimelate thioester reacts with L-alanine in a decarboxylative condensation catalyzed by BioF to form 8-amino-7-oxononanoate, the first intermediate in formation of the fused heterocyclic rings of biotin. Ring formation is completed by successive reactions catalyzed by BioA, BioD and BioB to generate biotin. (b) The BioW reaction with pimelate as substrate with separation of substrates and products by reverse phase HPLC. The absorbance peaks were identified using authentic standards of ATP, AMP and CoA. Below the chromatogram the steady state kinetic parameters for pimelate and CoA determined in sodium HEPES buffer pH 7 with 50 nM BioW are given. The parameters were determined by fitting the data from three independent experiments to Lineweaver-Burk plots and are given as the mean ± standard error. (c) Thin layer chromatography of α-32P-ATP labeled reactions in the absence and presence of CoA. A time course is shown for the CoA reactions. (d) Identification of pimeloyl-CoA (calculated mass 909.7) by electrospray ionization mass spectroscopy. The sharp peak eluting after 31 min in (b) was collected dried under nitrogen and submitted for mass spectroscopy. See Figs. S1, S2 and S3 for BioW purification and characterization.