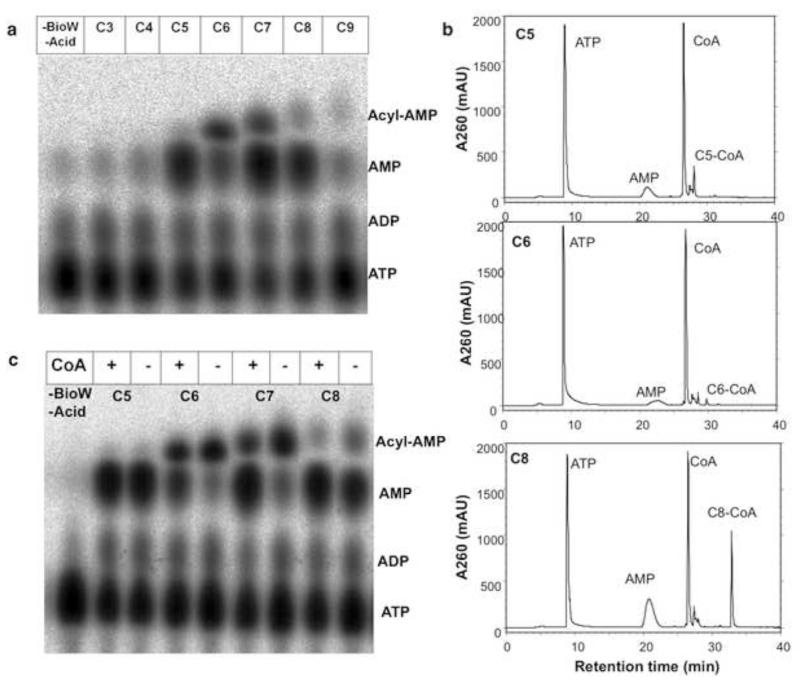
Figure 2. Dicarboxylate specificity of BioW.
(a) BioW reactions were performed with dicarboxylic acids from C3 to C9 to determine acyl chain substrate specificity. The reactions were carried out as described in Experimental Procedures and 1 μl of each reaction was spotted onto cellulose TLC plate. (b CoA thioester formation from the C5, C6 and C8 dicarboxylates after reaction with BioW at 37°C for 1 h. Panels from top to bottom show HPLC analyses of BioW reactions with the C5, C6 and C8 dicarboxylates, respectively. (c) Evidence of adenylate editing in absence of CoA. BioW reactions with the C5, C6, C7 and C8 dicarboxylates were carried out in presence or absence of CoA at 37°C for 1 h. Samples of 1 μl were spotted onto a TLC plate which was developed as described in Experimental Procedures. Note that the migration rates of the acyl-AMP intermediates vary with the dicarboxylate moiety. C8-CoA was identified by mass spectroscopy (Fig. S4) whereas the trace amounts of C5-CoA formed were insufficient for analysis.