Fig. 1.
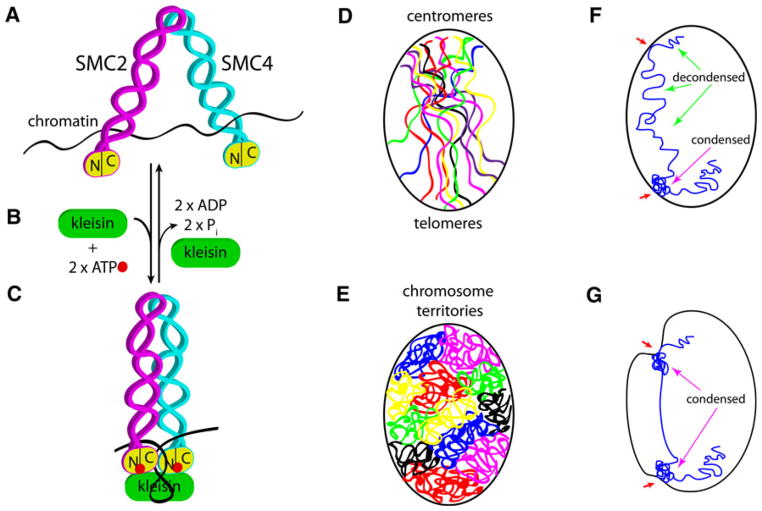
Condensins drive changes in chromosome organization and nuclear shape in interphase. a Eukaryotic condensin complexes consist of a heterodimer of two SMC proteins, SMC2 and SMC4. Each SMC subunit has half its ATPase on its N-terminus (N) and the other half on the C-terminus (C), which come together to form a functional ATPase “head,” shown in yellow. SMC proteins form a coiled-coil domain and a hinge domain, where dimerization occurs. SMC dimers can interact directly with DNA or chromatin (black line). Condensin not bound to ATP is thought to have an “open” conformation. b The SMC dimer can bind one ATP molecule (red) in each of the two ATPase heads, and ATP-bound head domains can then recruit a kleisin subunit (green). Barren/Cap-H is a condensin I-specific kleisin; Cap-H2 is a condensin II-specific kleisin. Other chromosome associated proteins (Cap) can also be recruited to the complex (not shown) in a condensn I- or II-specific manner. Additional Cap subunits are thought to mediate specific protein-protein interactions. See Table 1 for a complete list of SMC and Cap subunit genes. c ATP-bound SMC2/4 dimer induces a conformational change to a “closed” state. This conformational change is thought to drive axial shortening of chromosomes by inducing compaction of chromatin. Kleisin binding inhibits ATP hydrolysis and may serve to stabilize the closed SMC conformational state. High levels of kleisin favor the closed conformation. Dissociation of kleisin and ATP hydrolysis (as in b) reestablishes the open conformation and allows decondensation of chromatin. d Chromosomes (colored lines) are contained within the nuclear envelope (black oval) and exist in the Rabl conformation, where centromeres and telomeres are at opposite ends of the nucleus. Centromeres, telomeres, and other regions are thought to be tethered to the inner nuclear membrane through chromatin interactions with envelope-associated proteins. e Interphase chromosomes can adopt territories where each chromosome occupies a discrete position in the three-dimensional space of the nucleus. The position of each chromosome relative to the nuclear envelope and to other chromosomes is important for the expression of genes. It has been proposed that condensin II compaction forces in interphase are required for organizing chromosomes into territories. Chromatin tethers to the envelope are speculated to serve as anchors of condensin-driven gathering of chromatin as it condenses. f An interphase nucleus is shown with one chromosome (blue) for simplicity. Chromosomes can have regions that are relatively decondensed (green arrows) and condensed (magenta arrow) that reflect tissue-specific chromatin and gene expression states. Chromatin can be tethered to the inner nuclear membrane (red arrows). g A speculative model where local condensation states can be modulated, likely by local condensin activation, and envelope-tethered chromatin anchors may be drawn toward the interior of the nucleus. Invaginations and distortions of the envelope (red arrows) may result from local chromatin condensation or defects in nuclear envelope structure (see text for further details)