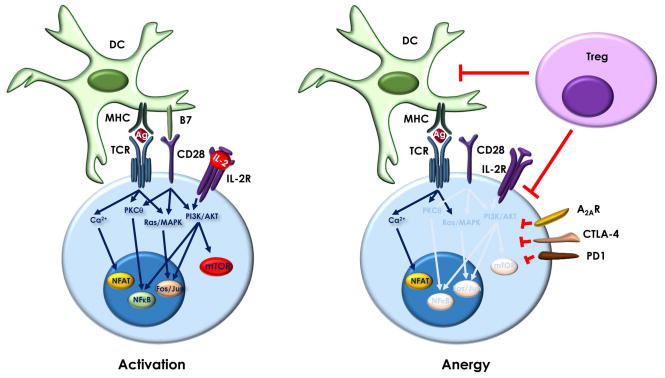
Figure 1.
Signal integration determines T cell fate. Activated T cells integrate signals triggered by recognition of MHC-antigen (Ag) complexes by the TCR, together with those induced by the engagement of CD28 by B7 ligands and by binding of IL-2 to the IL-2 receptor. Those signals translate into the activation of a series of signaling pathways, including increased calcium entry and activation of PKCθ, Ras/MAPKs, PI3K/AKT and mTOR, which allow the T cell to upregulate its metabolism and induce the transcription factors (e.g. NFAT, Fos/Jun or NFκB) required to maintain an activation-induced program of gene expression. When TCR engagement occurs in the absence of costimulation and/or the presence of inhibitory signals (e.g. effects of Tregs on DCs and effector T cells or engagement of coinhibitory receptors such as CTLA-4, PD-1 or A2aR) and unbalance activation of those signaling pathways leads to the induction of an alternative program of gene expression that will result in anergy.