Abstract
Scoring systems are used to assess the severity of a disease and the response to treatment. The main severity scoring indexes are the Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) and the Pemphigus Disease Area Index (PDAI). They have been validated and are already used in the evaluation of pemphigus and in clinical trials. They quantify disease severity by performing a global assessment of all lesions. In recent years, other severity scoring systems have been developed for pemphigus and other autoimmune blistering diseases.
Introduction
Autoimmune bullous diseases (AIBDs) consist of a heterogeneous group of rare cutaneous disorders that have various affects on quality of life.1 Disease severity fluctuates due to both the disease and treatment, making it necessary to have an objective scoring system to measure changes in severity. Pure subjective methods of assessing disease severity have significant variation, and are plagued with problems, as they are unreliable, inaccurate, and inconsistent. Scoring systems have, therefore, been developed to provide objective, standardized values to reflect disease severity. These scoring systems enable the clinician to monitor the condition and modify treatment according to severity. Quantifying disease severity provides an opportunity to perform interpatient and intrapatient comparisons and to assess the effectiveness of therapeutic regimens. The differing accuracy of these scoring systems not only depends on the scoring system itself but also the underlying disease. Each scoring system should be validated to ensure it truly correlates with disease activity. Interobserver and intraobserver variability can be minimized by training investigators in how to properly use the scoring system.2 Due to the rarity of the disease, there is a paucity of randomized controlled trials (RCTs). Even the RCTs that do exist have large variations in quality, are not well designed, and provide results that are often uninterpretable.3,4 Different outcome measures and end points make direct comparisons between studies impossible. The development of definitions of disease, therapeutic response, and objective scoring systems has provided opportunities for direct comparisons between various treatment regimens in RCTs.5
Autoimmune bullous skin disorder intensity score
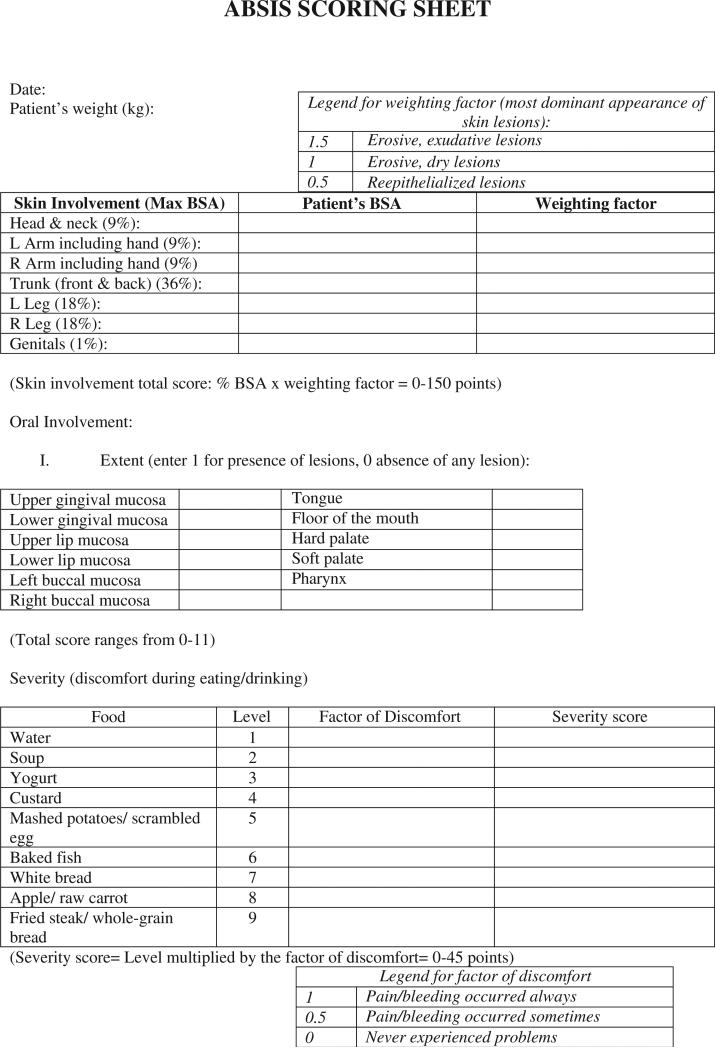
The Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) was developed in 2007 as a scoring system to measure and capture changes in disease severity for pemphigus.2 The clinical presentation of pemphigus is varied and a scoring system to quantify small changes in disease severity was necessary to compare the efficacy of medications. The ABSIS, a scoring system with a maximum score of 206, uses the rule of 9s, which is used in burns measurement, to assess the percentage of involvement of blisters and erosions on the skin combined with a weighting factor for the stage of the blistering and erosions, respectively (Figure 1).2 The cutaneous involvement score consists of 2 parts: percentage of involvement (body surface area [BSA]) and the quality of lesions. Each body part is assumed to be 9% or a multiple of 9%, such that in adults the head and neck is 9%, one arm (including the hand) is 9%, the trunk is 36%, one leg is 18%, and the genitals are 1%. It is assumed the patient's palm is 1% of BSA. The quality of lesions is assessed by multiplying the extent of BSA by a weighting factor. Erosive, exudative lesions, and positive Nikolsky's sign obtain a weighting factor of 1.5; erosive, dry lesions have a weighting factor of 1.0; and reepitheliazed lesions (excluding postinflammatory erythema and/or hyperpigmentation) have a weighting factor of 0.5. The predominant quality of the lesions within the respective anatomical region (ie, trunk, upper and lower extremities) determines the weighting factor to be used. Oral involvement is based on 2 scores comprising the extent (presence of lesions) and severity (discomfort during eating and drinking) of the disease. The extent is given a score of 0 or 1 (absence or presence, respectively) for 11 different parts of the mouth.7 These 11 sites are upper and lower gingival mucosae, upper and lower lip mucosae, left and right buccal mucosae, the tongue, floor of the mouth, hard and soft palate, and the pharynx. The severity of oral lesions is assessed by the amount of pain/bleeding associated with certain foods. The factor discomfort is attributed a score of 0, 0.5, or 1 for the symptoms of never experiencing problems, pain/bleeding occurring sometimes, or pain/bleeding occurring always, respectively. The final severity score is the summation of the products of the food-specific score with the factor discomfort value. The maximum scores for oral involvement are 11 for extent and 45 for severity.
Fig. 1.
The advantage of the ABSIS is that it provides both qualitative and quantitative information. The oral involvement scores comprise both objective and subjective information. Subjective qualities such as relief of pain on eating may not be detected by a clinician who is solely assessing the number of oral lesions. Complete subjective scores in which patients focus on their symptoms (eg, oral pain) would not take into consideration the cutaneous lesions that are of importance to the clinician.7 Therefore, the combination of both subjective and objective information makes the ABSIS superior to other scoring systems.
Pemphigus disease area index
Another major scoring system is the Pemphigus Disease Area Index (PDAI), which was developed by the International Pemphigus Definitions Committee over a 3-year period5 (Figure 2). It has 3 components relating to the skin, scalp, and mucous membranes. The skin has activity and damage scores. The activity score is a value given to the number of erosions, blisters, or new erythema at the time of examination. An individual activity score for the 12 anatomic locations is obtained by assigning a score for each site based on fitting into a bracketed number of lesions and sizes. An actual lesion count was not used because they are notoriously inaccurate. Only numbers less than 3 in one site are specifically counted and otherwise the diameter of the largest lesion can elevate the grading of a site into a higher group, because coalescing smaller blisters could lower the absolute number of blisters but enlarge the size, a sign of severity of pemphigus. The summation of individual scores provides a final activity score, which is out of 120. The damage score is 1 if postinflammatory hyperpigmentation or erythema from a resolving lesion is present and 0 otherwise. A similar approach for 12 parts of the mucous membranes results in a mucous membrane severity score out of 120. The scalp activity depends on the number of quadrants affected, with a maximum score of 10. The total maximum score is 263, consisting of 250 points from the activity and 13 from damage scores.
Fig. 2.
PDAI scoring sheet developed by the International Pemphigus Definitions Group with the support of the International Pemphigus and Pemphigoid Foundation.5,6
A recent study examining the reliability and convergent validity of ABSIS and PDAI had 10 trained dermatologists assess 15 patients with pemphigus using the 2 severity scores.6 The score obtained by using the PDAI represented the true clinical severity and was more reproducible than the ABSIS. The mean length of time to complete the PDAI was 4.7 minutes compared with 3.9 minutes for the ABSIS. Although the inter-rater reliability was similar, the intra-rater reliability was higher in PDAI than ABSIS (0.98 vs 0.80, respectively); however, it is suggested the subjective component of the ABSIS contributed to its high inter-rater reliability. The PDAI was able to detect smaller differences in severity in moderate-to-severe pemphigus. It may be considered a reliable tool to use in clinical trials, due to its accuracy in assessing small differences in patients with mild disease or who have responded to treatment, and can be used as a means to distinguish between various therapeutic options.6 However, the cohort investigated in the study by Rosenbach et al.6 mainly consisted of mild-to-moderate pemphigus patients, leading to a critical bias of this comparative study. Future multicenter studies investigating larger numbers of patients with variable disease activities including severe cases are mandatory to finally evaluate the differences of these 2 scoring systems.
ABSIS has been recently used as a clinical parameter in a retrospective study that compared the efficacy of adjuvant treatment with immunoadsorption or rituximab, respectively, in patients with recalcitrant pemphigus.8 Both adjuvant treatments led to a rapid decrease of ABSIS skin and mucosa scores, which was associated with a decline of desmoglein-3– and desmoglein-1–specific immunoglobulin G. Similarly, ABSIS had been previously applied as an outcome measure in 2 patients with refractory epidermolysis bullosa acquisita who were treated with immunoadsorption followed by rituximab.9
Both, PDAI and ABSIS will be applied as clinical outcome measures in 3 prospective multicenter trials aimed at validating the therapeutic efficacy of rituximab, adjuvant immunoadsorption, and intravenous immunoglobulins (IVIG) in pemphigus. These clinical trials will hopefully help to identify the relative strength of ABSIS and PDAI as clinical outcome measures.
Others
Other less common scoring systems have been developed and are described as follows. Mahajan et al10 assessed the severity of pemphigus by the degree of BSA involved by classifying patients as mild, moderate, severe, or extensive. This classification, although easy to use, has broad, simplified categories that would not detect gradual changes in disease severity as clinicians and patients may have different views on what constitutes mild, moderate, severe, or extensive. A significant change in BSA involvement would be needed to shift a patient from a moderate severity score (defined as “10%-25% BSA involvement along with oral mucosal involvement. Able to carry out daily routine with discomfort”) to a severe severity score (defined as “25%-50% BSA involvement along with oral mucosal involvement; unable to carry out daily routine with discomfort”). Therefore, it is imprecise and would not capture small changes in the severity score in clinical studies.
The pemphigus area and activity score (PAAS)11 is based on the psoriasis area and severity index (PASI). It is used for patients with cutaneous pemphigus with an additional component if mucous membranes are involved. The body is divided into 4 areas (head, trunk, upper limbs, and lower limbs) with associated weights of 0.1, 0.3, 0.2, and 0.4, respectively. The score is obtained by considering the number of new blisters, peripheral extension of existing blisters, Nikolsky's sign, and the percentage of area affected. A subsequent study found that there was a statistically significant correlation between the mucosal and cutaneous severity scores obtained from the PAAS and antibody titers based on indirect immunofluorescence.12 A major criticism of the PAAS is that large changes in BSA are required to reflect change in the severity score.2 Also, counting the number of new blisters does not necessarily correlate with symptoms, mortality, or the size of the lesions and, therefore, is not an accurate measure of disease activity.
The Ikeda index was first developed in 1993 with subsequent revisions in 1997 and 2000.13 A score of 0 to 3 is given to 4 domains (affected area %, Nikolsky phenomenon, number of new blisters per day, oral lesions %) to give a severity score ranging between 0 and 12. It has been assessed in Japanese studies14 but is also imprecise. The Nikolsky assessment is subjective.
Saraswat et al7 developed a scoring system for oral pemphigus in 2003, which was later incorporated into the ABSIS system. This scoring system is not limited to pemphigus and it has been suggested it can be used in other conditions such as mucous membrane pemphigoid, Stevens Johnson Syndrome, and herpetic gingivostomatitis.7
Another possible severity scoring system is to base the score on the level of corticosteroid and/or adjuvant use. Because most patients with AIBD are commenced on corticosteroids at some point, measuring the actual or change in dose may reflect the change in severity of the disease.15 This imprecise system is dependent on many factors including the clinician's willingness to prescribe medications, comorbidities, and the effectiveness of the treatment in that condition. For example, there is evidence to suggest topical corticosteroids are more effective than systemic corticosteroids in the management of bullous pemphigoid,16 demonstrating this scoring system cannot be applied to all AIBDs.
The use of desmoglein-1 and -3 antibody levels by enzyme-linked immunosorbent assay (ELISA) to monitor the severity of pemphigus is controversial. Although some studies have demonstrated a relationship between disease severity and these levels,17-21 there are others that show the antibodies do not correlate with the clinical course and take a long time to decrease despite clinical improvement.22
Physician's global assessment (PGA) is a visual analogue scale that has been used in clinical trials because it is fast and easy to use.1,23 The crude score obtained is not as reliable and accurate as the ABSIS or PDAI.6
A severity scoring system involving ocular manifestations in AIBD was developed by Tauber et al24 to improve on previous scoring systems25,26 that were not as sensitive in detecting changes in severity. This system assessed the degree of symblepharon or fornix foreshortening and became a more sensitive tool in determining progression. A newer method,27 which incorporates the distance between the lower limbus and a specific location of the eyelid margin in different gaze positions detects subtle changes of disease progression compared with the system proposed by Tauber et al.24
Quality of life in AIBD
The paper by Sebaratnam et al., elsewhere in this issue, addresses studies and measures of quality of life in AIBD, which are also relevant in assessing the severity of an AIBD.
Conclusions
The development of severity scoring systems has done away with vague classifications of severity and provides a numerical value that can be used to make accurate and meaningful comparison between patients. It allows greater comparison between small trials, as AIBDs are rare, using meta-analysis to determine the efficacy of treatment regimens and these scores equip clinicians and government authorities with evidence to support particular medications. Before the introduction of these severity scoring systems, the definitions of remission were varied and confusing.2,28 The ABSIS and PDAI are the 2 main validated severity scoring systems for pemphigus, which can also be used in other AIBDs. Further validation studies are required to assess the accuracy of the other scoring systems.
Acknowledgment
This work was supported in part by the National Institutes of Health (grant number NIH K24-AR 02207) to VPW.
References
- 1.Tabolli S, Mozzetta A, Antinone V, Alfani S, Cianchini G, Abeni D. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029–34. doi: 10.1111/j.1365-2133.2008.08481.x. [DOI] [PubMed] [Google Scholar]
- 2.Pfutze M, Niedermeier A, Hertl M, Eming R. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus. Eur J Dermatol. 2007;17:4–11. doi: 10.1684/ejd.2007.0090. [DOI] [PubMed] [Google Scholar]
- 3.Martin LK, Murrell DF. Treatment of pemphigus: the need for more evidence. Arch Dermatol. 2008;144:100–1. doi: 10.1001/archdermatol.2007.14. [DOI] [PubMed] [Google Scholar]
- 4.Martin LK, Werth V, Villanueva E, Segall J, Murrell DF. Interventions for pemphigus vulgaris and pemphigus foliaceus. Cochrane Database Syst Rev. 2009:CD006263. doi: 10.1002/14651858.CD006263.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Murrell DF, Dick S, Ahmed AR, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. 2008;58:1043–6. doi: 10.1016/j.jaad.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbach M, Murrell DF, Bystryn JC, et al. Reliability and convergent validity of two outcome instruments for pemphigus. J Invest Dermatol. 2009;129:2404–10. doi: 10.1038/jid.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saraswat A, Bhushan K, India C. A new grading system for oral pemphigus. Int J Dermatol. 2003;42:413–4. doi: 10.1046/j.1365-4362.2003.01811.x. [DOI] [PubMed] [Google Scholar]
- 8.Pfutze M, Eming R, Kneisel A, Kuhlmann U, Hoyer J, Hertl M. Clinical and immunological follow-up of pemphigus patients on adjuvant treatment with immunoadsorption or rituximab. Dermatology. 2009;218:237–45. doi: 10.1159/000187431. [DOI] [PubMed] [Google Scholar]
- 9.Niedermeier A, Eming R, Pfütze M, et al. Clinical response of severe mechanobullous epidermolysis bullosa acquisita to combined treatment with immunoadsorption and rituximab (anti-CD20 monoclonal antibodies). Arch Dermatol. 2007;143:192–8. doi: 10.1001/archderm.143.2.192. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan VK, Sharma NL, Sharma RC, Garg G. Twelve-year clinico-therapeutic experience in pemphigus: a retrospective study of 54 cases. Int J Dermatol. 2005;44:821–7. doi: 10.1111/j.1365-4632.2005.02218.x. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal M, Walia R, Kochhar AM, Chander R. Pemphigus Area and Activity Score (PAAS)—a novel clinical scoring method for monitoring of pemphigus vulgaris patients. Int J Dermatol. 1998;37:158–60. [PubMed] [Google Scholar]
- 12.Mortazavi HK, Esmaili K, Chams-Davatchi N. Correlation of pemphigus vulgaris antibody titers by indirect immunofluorescence with activity of disease based on pemphigus area and activity score (PAAS). Acta Medica Iranica. 2008;46:239–44. [Google Scholar]
- 13.Ikeda S, Imamura S, Hashimoto I, Morioka S, Sakuma M, Ogawa H. History of the establishment and revision of diagnostic criteria, severity index and therapeutic guidelines for pemphigus in Japan. Arch Dermatol Res. 2003;295:S12–6. doi: 10.1007/s00403-002-0367-2. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa H, Sakuma M. Annual Report of the Intractable Skin Diseases Study Group. Ministry Welfare Health Tokyo; Japan: 1995. A nation-wide questionnaire investigation about severity and treatment of pemphigus (second report). pp. 251–61. [Japanese] [Google Scholar]
- 15.Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol. 2000;42:422–7. doi: 10.1016/s0190-9622(00)90213-5. [DOI] [PubMed] [Google Scholar]
- 16.Joly P, Roujeau JC, Benichou J, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346:321–7. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 17.Chorzelski TP, Von Weiss JF, Lever WF. Clinical significance of autoantibodies in pemphigus. Arch Dermatol. 1966;93:570–6. [PubMed] [Google Scholar]
- 18.Harman KE, Seed PT, Gratian MJ, Bhogal BS, Challacombe SJ, Black MM. The severity of cutaneous and oral pemphigus is related to desmoglein 1 and 3 antibody levels. Br J Dermatol. 2001;144:775–80. doi: 10.1046/j.1365-2133.2001.04132.x. [DOI] [PubMed] [Google Scholar]
- 19.Herrero-Gonzalez JE, Iranzo P, Benítez D, Lozano F, Herrero C, Mascaró JM., Jr Correlation of immunological profile with phenotype and disease outcome in pemphigus. Acta Derm Venereol. 2010;90:401–5. doi: 10.2340/00015555-0868. [DOI] [PubMed] [Google Scholar]
- 20.Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–7. [PubMed] [Google Scholar]
- 21.Schmidt E, Dähnrich C, Rosemann A, et al. Novel ELISA systems for antibodies to desmoglein 1 and 3: correlation of disease activity with serum autoantibody levels in individual pemphigus patients. Exp Dermatol. 2010;19:458–63. doi: 10.1111/j.1600-0625.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick RE, Newcomer VD. The correlation of disease activity and antibody titers in pemphigus. Arch Dermatol. 1980;116:285–90. [PubMed] [Google Scholar]
- 23.Guzzo CA, Weiss JS, Mogavero HS, et al. A review of two controlled multicenter trials comparing 0.05% halobetasol propionate ointment to its vehicle in the treatment of chronic eczematous dermatoses. J Am Acad Dermatol. 1991;25:1179–83. doi: 10.1016/0190-9622(91)70322-s. [DOI] [PubMed] [Google Scholar]
- 24.Tauber J, Jabbur N, Foster CS. Improved detection of disease progression in ocular cicatricial pemphigoid. Cornea. 1992;11:446–51. doi: 10.1097/00003226-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Mondino BJ, Brown SI. Ocular cicatricial pemphigoid. Ophthalmology. 1981;88:95–100. doi: 10.1016/s0161-6420(81)35069-6. [DOI] [PubMed] [Google Scholar]
- 26.Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc. 1986;84:527–663. [PMC free article] [PubMed] [Google Scholar]
- 27.Rowsey JJ, Macias-Rodriguez Y, Cukrowski C. A new method for measuring progression in patients with ocular cicatricial pemphigoid. Arch Ophthalmol. 2004;122:179–84. doi: 10.1001/archopht.122.2.179. [DOI] [PubMed] [Google Scholar]
- 28.Vaishnani JB, Bosamiya SS. Pemphigus: active or inactive? Indian J Dermatol. 2009;54:186–8. doi: 10.4103/0019-5154.53173. [DOI] [PMC free article] [PubMed] [Google Scholar]