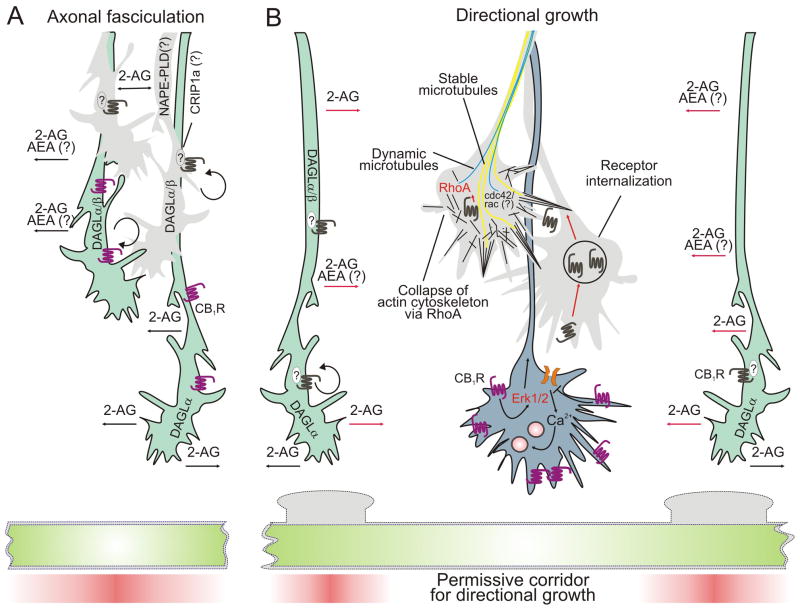
Fig. 3. Unifying concept defines endocannabinoid-driven spatial segregation of inhibitory and excitatory synapses.
(A) Pyramidal cells emit the first axons in cerebral circuits. They exhibit moderate CB1R expression with these receptors distributed all along the axis of the elongating axon. Sn-1-diacylglycerol lipases (DAGLα/β) are co-expressed in excitatory axons permitting cell-autonomous endocannabinoid (eCB) signaling that drives axonal elongation. Target-derived 2-arachidonoylglycerol (2-AG) might act as an additional attractive force. (B) Later-arriving GABAergic axons do not express DAGLs in their growth cones, but do express high levels of CB1Rs that sense a microenvironment that contains hotspots of 2-AG emanating from excitatory afferents (red shading). In these neurons, upon eCB stimulation, CB1Rs are removed from motile filopodia and translocated to the central growth cone domain where they activate the extracellular signal-regulated kinase 1/2 (Erk1/2) pathway, and RhoA GTPases. RhoA activation leads to a collapsing response on the side of the growth cone facing the eCB gradient thus contributing to growth cone steering decisions. Consequently, GABAergic axons target specific dendritic domains of postsynaptic neurons whose subcellular distribution and size are defined by excitatory afferents. Abbreviations: AEA, 2-arachidonoylethaloamine/anandamide; CB1R, CB1 cannabinoid receptor; CRIP1a, cannabinoid receptor interacting protein 1a; NAPE-PLD, N-acyl-phosphatidylethanolamine-selective phospholipase D; RhoA/Cdc42/Rac, members of the Rho family of small GTPases