Figure 8. Secondary structure timeline of the cTnI-Rr/switch.
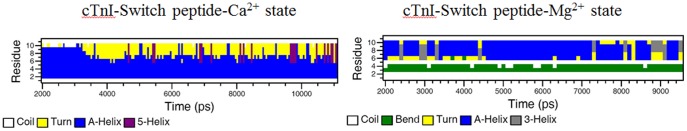
The cTnI-Rr is held within the cTnC N-domain hydrophobic pocket. In the presence of regulatory Ca2+ the cTnI-Rr maintains a helical conformation. In the absence of regulatory Ca2+ the secondary structure is perturbed. The absence of the helical conformation would release the cTnI-Md to interact with actin in the Ca2+-free state whereas, the presence of the regulatory Ca2+ would refold the cTnI-Rr into a helix effectively retracting the cTnI-Md from actin.
