Fig. 5.
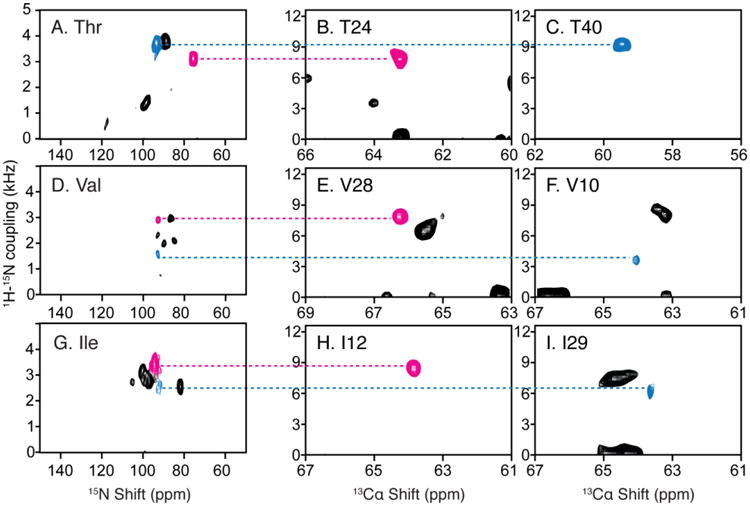
Resonance assignments of residues in OS solid-state NMR spectra utilizing the 1H-15N dipolar couplings measured in RA MAS solid-state NMR experiments. A. D. and G. Two-dimensional SLF spectrum of amino-acid-type selectively 15N labeled MerF in ‘unflipped’ DMPC/DHPC bicelles. B. C. E. F. H. and I. Two-dimensional SLF spectral planes extracted from a three-dimensional HnNCa experiment, of which the third dimension is 15N chemical shift (Lu et al. 2013). The scales during aligning the 1H-15N dipolar couplings are adjusted for the scaling factors of the bicelles vs. liposomes (0.8) and of the alignment perpendicular to the magnetic field vs. rotational alignment parallel to the bilayer normal (0.5).
