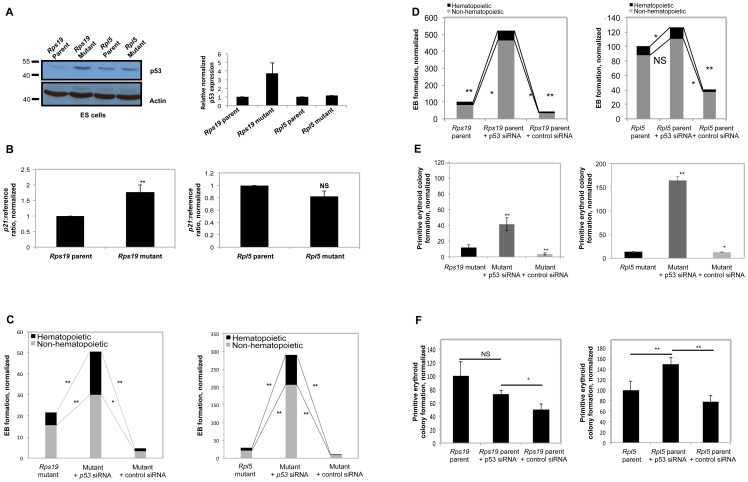
Figure 4. The differentiation defects observed in Rps19 and Rpl5 mutants are nonspecifically rescued by p53 inhibition.
(A) Western blot analyses were performed from mutant ES cells with antibodies against p53, using β-Actin as a loading control. ES cells from the Rps19 mutant cells showed an increase in p53 expression. In contrast, the Rpl5 mutant expressed no increase in p53, compared with the parental line. Image J quantification of western blots from 3 independent experiments demonstrated that the Rps19 mutant ES cells had approximately a 4-fold increase in p53 protein compared to the wild type cells. (B) qRT-PCR performed on these ES cells showed an increase in p21 mRNA only in the Rps19 mutant ES cells (3 independent experiments) while there was no similar increase in the Rpl5 mutant ES cells (4 independent experiments). siRNA targeting p53 was used to transiently transfect ES cells 24 hours prior to primary differentiation, obtaining >90% p53 knockdown by qRT-PCR. Both mutants (C) showed a significant increase in EB formation with p53 knockdown (4 independent pooled experiments). This effect was nonspecific, as p53 knockdown of parental cells also increased EB formation (D). The primitive erythroid colony defect was partially compensated in the Rps19 mutant after p53 inhibition and overcompensated in the Rpl5 mutant (E) (3 independent pooled experiments). This augmentation of colony formation was again nonspecific, as there was an increase in primitive colony formation with p53 knockdown in both parental ES cells when compared with the control siRNA (3 independent pooled experiments for Rpl5 parent and 4 independent experiments for Rps19 parent) (F).