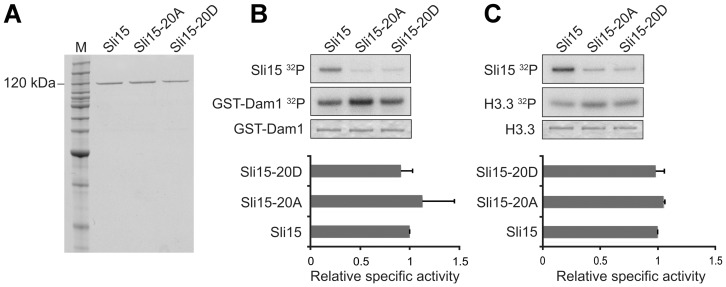
Figure 2. In vitro Ipl1-dependent phosphorylation promoted by wild-type and mutant versions of Sli15.
(A) Coomassie-stained gel showing recombinant GST-Sli15-His6 preparations used for in vitro phosphorylation and microtubule binding. (B) Phosphorylation of GST-Dam1 by Ipl1 in the absence or presence of wild-type (Sli15) or mutant forms (Sli15-20A, Sli15-20D) of GST-Sli15-His6. Top panel, GST-Sli15-His6 phosphorylation (32P incorporation from [γ-32P]ATP); middle panel, GST-Dam1 phosphorylation (32P incorporation from [γ-32P]ATP); lower panel, GST-Dam1 detected by Coomassie staining. (C) Phosphorylation of H3.3 by Ipl1 in the absence or presence of wild-type or mutant forms of GST-Sli15-His6. Top panel, GST-Sli15-His6 phosphorylation (32P incorporation from [γ-32P]ATP); middle panel, H3.3 phosphorylation (32P incorporation from [γ-32P]ATP); lower panel, H3.3 detected by Coomassie staining. The histograms show mean relative specific activity (n = 3) of H3.3 (B) and GST-Dam1 (C) phosphorylation by Ipl1 promoted by GST-Sli15-20A-His6 or GST-Sli15-20D-His6 in comparison with GST-Sli15-His6 (arbitrarily set to 1.0).