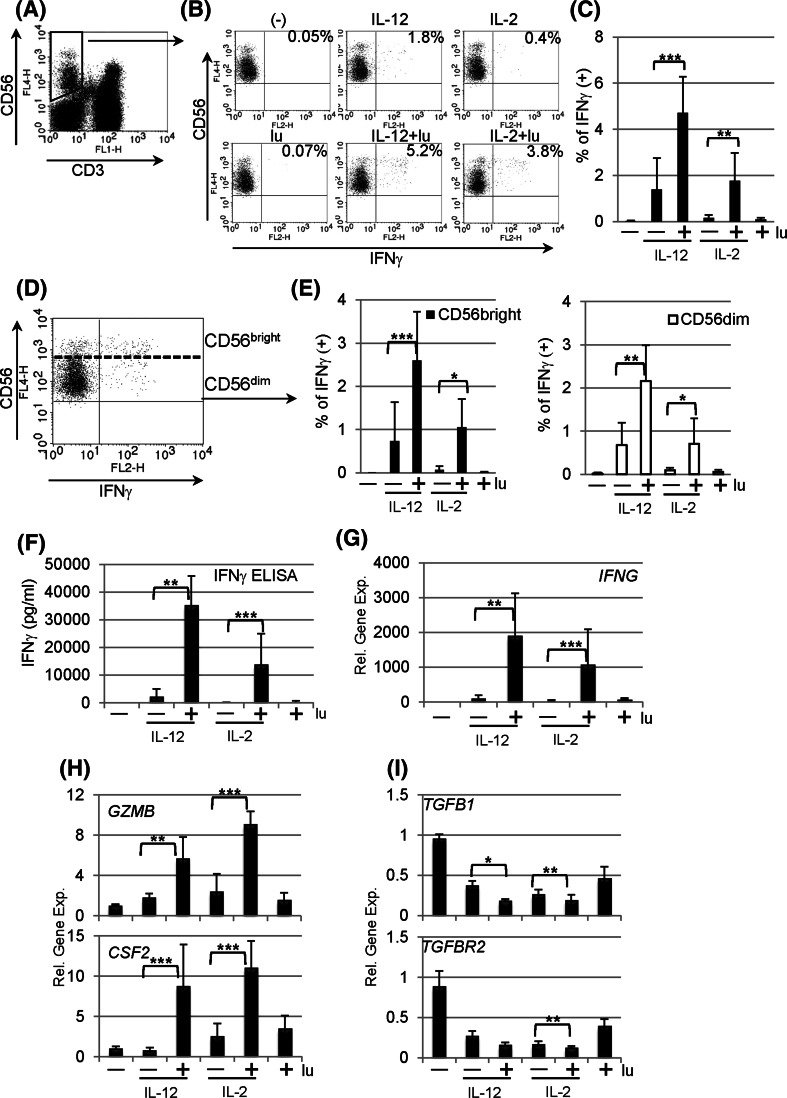
Fig. 1.
Lunasin stimulates human peripheral NK cells. Peripheral blood mononuclear cells (PBMCs) of normal controls were stimulated with medium only (−), lunasin at 20 μM (lu), cytokine IL-12 at 10 ng/ml or IL-2 at 100 U/ml, and cytokine plus lunasin for 24 h. The lunasin peptide was chemically synthesized by LifeTein (South Plainfield, NJ). The production of IFNγ at single-cell levels was analyzed using intracellular cytokine staining (a–e). At the last 6 h of stimulation, golgistop (monensin) was added to block the secretion of IFNγ. Stimulated PBMCs were surface stained with FITC-conjugated CD3 and APC-conjugated CD56 monoclonal antibodies, washed, fixed, and permeabilized. After washing, cells were incubated with PE-conjugated anti-IFNγ monoclonal antibody. Expression of IFNγ was evaluated using flow cytometry on 5,000 events of gated CD3 negative and CD56 positive NK cell populations (a). A representative dot plot from one donor shows the percentage of IFNγ producing NK populations following various treatments (b), and the averaged percentage of IFNγ producing NK populations are presented as mean ± SD from 5 different normal donors (c). IFNγ producing NK cells are further segregated into CD56 bright and CD56 dim populations (d), and the percentage of IFNγ producing CD56 bright or CD56 dim populations is averaged from the same 5 donors as in c and presented as mean ± SD (e). f The secretion of IFNγ by purified NK cells following stimulation was analyzed using ELISA. Freshly isolated human NK cells from PBMCs of normal controls using positive selection with CD56 magnetic beads (Miltenyi Biotec, Auburn, CA) were stimulated as in b. Following 1 day of stimulation, cell-free supernatants were evaluated for IFNγ production, and data are presented as mean ± SD averaged from 5 different controls. g–i Effects of lunasin on gene expression by human NK cells. The cell pellets collected from f were resuspended in Trizol Reagents for total RNA extraction. The first-strand cDNA was synthesized followed by real-time qPCR using Taqman assay with primers for g IFNγ (IFNG), h granzyme B (GZMB) and granulocyte–macrophage colony-stimulating factor (GM-CSF or CSF2), and i TGFβ (TGFB1) and TGFβ receptor (TGFBR2) in ABI 7300 (Applied Biosystems by Life Technologies, Carlsbad, CA). Data are presented as mean ± SD averaged from 5 different controls. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001