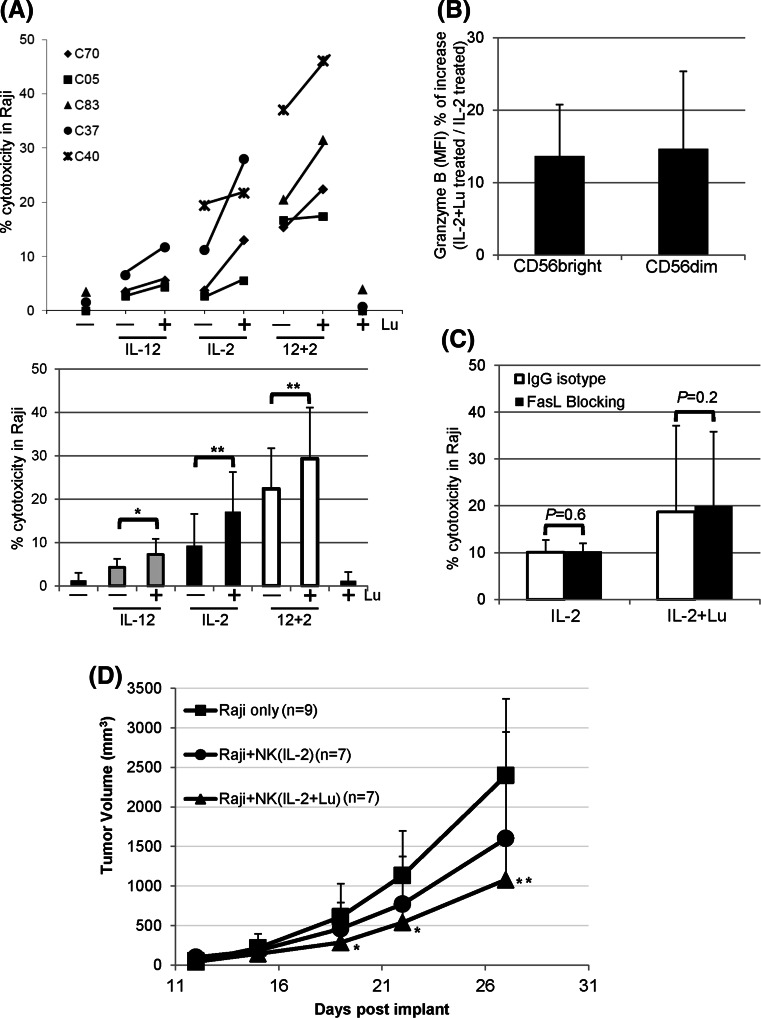
Fig. 4.
Effects of lunasin on NK cell-mediated cytotoxicity against human B lymphoma Raji cell line. a NK-mediated cytotoxicity in vitro. Freshly isolated human NK cells from PBMCs of normal controls using negative selection (Miltenyi Biotec) were stimulated with medium only (−), single or both cytokines (IL-12 and IL-2) without (−) or with (+) lunasin (20 μM), or lunasin alone for 1 day. Cytokines were used at suboptimum concentrations of 1 ng/ml for IL-12 and 10 U/ml for IL-2. The in vitro cytotoxicity was performed using lactate dehydrogenase (LDH)-releasing assay with the CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (Promega, Madison, WI). The effectors were co-cultured with target cells at ratio of 10:1 for 4 h at 37 °C in a 5 % CO2 incubator. The percentage of cytotoxicity was calculated according to the manufacturer’s instructions. Each symbol represents an individual donor, and the percentage of cytotoxicity from each donor (n = 5, C70, C05, C83, C37, and C40) following various treatments is depicted in the upper panel of a. The averaged percentage of cytotoxicity from 3 to 4 donors at each treatment is presented as mean ± SD in the lower panel of a. A mixed model including 6 levels of treatments was developed to analyze the data with within-subject treatments, and the pairwise comparisons among the treatments were performed to determine the P values. *P ≤ 0.05; **P ≤ 0.01. b Intracellular granzyme B staining from activated NK cells. Purified NK cells as in a were stimulated with suboptimum concentration of IL-2 (10 U/ml) alone or with lunasin (20 μM) for 1 day followed by intracellular staining for granzyme B. The level of granzyme B expression was analyzed from CD56 bright and CD56 dim NK populations, and the geometric mean fluorescent intensity (MFI) was obtained using flow cytometry. The MFI of bright and dim populations treated with IL-2+ lunasin was compared to IL-2, and the percentage of increase in granzyme B expression is presented as Mean ± SD averaged from 3 controls (b). c FasL-mediated killing by activated NK cells. Purified NK cells were stimulated as in b for 1 day followed by incubation with blocking antibody against FasL (filled bar) or IgG isotype control (open bar) for 2 h. FasL-mediated killing by activated NK cells was determined using the in vitro cytotoxicity assay as in a. The percentage of cytotoxicity against Raji cells is presented as mean ± SD averaged from 2 controls. d NK-mediated cytotoxicity in a human Raji lymphoma xenograft model. NOD/SCID/gcnull (NSG) mice at 2 months old were injected subcutaneously on day 1 with 0.5 × 106 Raji cells in 0.1 ml PBS mixed with 0.1 ml Matrigel (BD Biosciences, San Jose, CA). NK cells were isolated from the leucopack of donors and treated with IL-2 (10 U/ml) or IL-2+ lunasin (lu, 20 uM) for 1 day. On day 2, these treated NK cells were washed and injected into the tumor site (2.5 × 106 NK cells/mouse). Tumor growth was monitored, and the volumes were measured using standard manual calipers. Tumor volume (mm3) is presented as mean ± SD from 9 mice in the control group of Raji (no NK), and from 7 mice in the groups of Raji + NK (IL-2), and Raji + NK (IL-2+ lunasin). A mixed model with repeated measure to the data was developed using PROC MIXED in SAS program followed by pairwise comparison test of the mean differences among treatments by different days. *P ≤ 0.05; **P ≤ 0.01; relative to the control group of Raji (no NK)