Fig. 6.
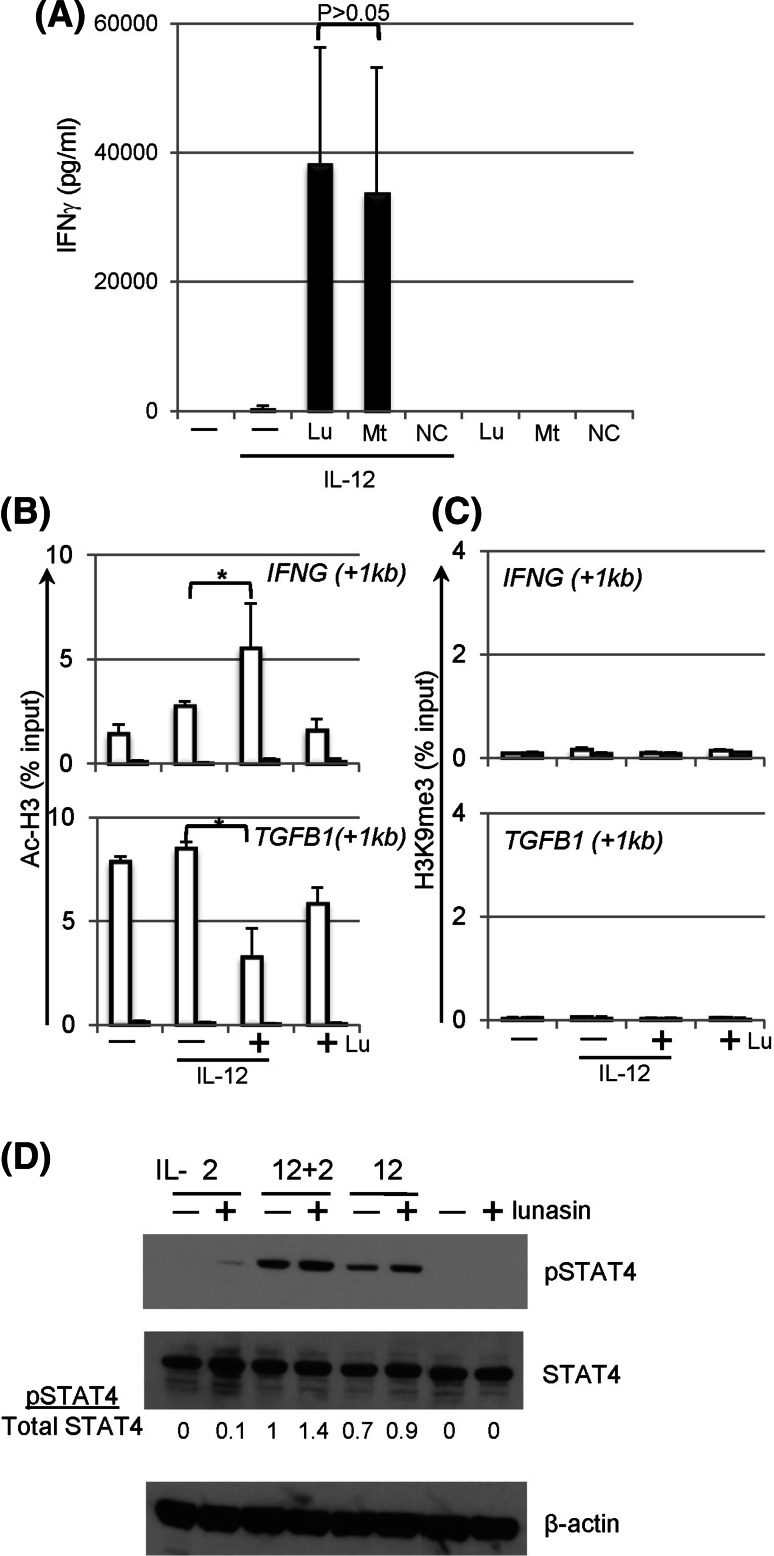
Mechanisms of synergistic effects mediated by lunasin. a The RGD motif and poly-D tails are not required for the synergistic effects of lunasin on IFNγ production by NK cells. Freshly isolated human NK cells (described in Fig. 1f) were stimulated with medium only (−), IL-12 (10 ng/ml) without (−) or with (+) the full-length lunasin (Lu), mutant peptide (Mt) lacking the RGD motif and poly-D tail, or negative control (NC) peptide with scrambled amino acids as well as peptides only as indicated. The concentrations of the peptides used were 20 μM. One day following stimulation, the production of IFNγ in the supernatants was determined using ELISA. Data are presented as mean ± SD averaged from 2 different normal controls. b, c Chromatin remodeling at the loci of target genes. Freshly isolated human NK cells (described in Fig. 1f) were stimulated with medium only (−), IL-12 (10 ng/ml) without (−) or with (+) lunasin (20 μM), or lunasin alone. Following 1 day of stimulation, cells were subjected to the ChIP assay. Chromatin DNA fragments were immunoprecipitated with antibodies against acetyl-histone H3 (AcH3) (b) and histone H3 trimethyl Lys9 (H3K9me3) (c) along with non-immune rabbit serum (filled bars) (Millipore, Billerica, MA), individually. The relative degree of histone modification of IFNG and TGFB1 loci was compared by qPCR using ChIP qPCR Primer Assay for human IFNG (+1 kb) and TGFB1 (+1 kb), respectively (SABioscience Qiagen, Valencia, CA). For calculation of ChIP results, the amount of immunoprecipitated DNA is normalized to the input chromatin in each reaction as a percentage of input (% input). Data are shown as mean percentage of input ± SD averaged from 3 controls. *P ≤ 0.05. d STAT4 activation in lunasin-cultured NK cells. Freshly isolated human NK cells from PBMCs of normal controls (described in Fig. 1f) were stimulated with single cytokine IL-2 (10 U/ml) or IL-12 (1 ng/ml), or both cytokines in the absence (−) and presence (+) of lunasin (20 μM) as well as medium and lunasin only. Activation of STAT4 was determined using Western blot of total protein extracts from cultured NK cells following 22 h of stimulation. Ratios of phospho-STAT4 to total STAT4 (pSTAT4/total STAT4) were determined from the arbitrary units of densitometry using the NIH ImageJ program as indicated. An anti-β-actin monoclonal (SC-47778) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used for the loading control. Results shown are representative from 2 different normal controls with similar profiles